Abstract
Available targeted therapies for colorectal cancer (CRC) are limited. Immunotherapy offers new options for cancer treatment, but most of CRC are refractory to current immune checkpoint blockade, which indicates the possible presence of yet uncharacterized immune-suppressive mechanisms. Herein we report that high levels of Dickkopf-related protein 2 (DKK2) are expressed in human CRC tumors, and the DKK2 blockade caused stronger activation of tumor-infiltrating CD8+ T cells in ex vivo culture. A correlation of high DKK2 expression with increased lymph node metastasis prevalence was observed in these CRC patients as well. Furthermore, antibody-mediated ablation of DKK2 activates natural killer (NK) cells and CD8+ T cells in tumors, impedes tumor progression, and reduced angiogenesis in a mouse genetic CRC model with mutations in APC and KRAS, which mimics advanced human CRC. Based on this, we performed a combined administration of DKK2 blockade with sub-optimal VEGFR blockade and observed a synergetic effect on suppressing tumor angiogenesis and progression, as well as extending survival, which yielded better results than those of every single therapy. Thus, this study provides further evidence for the potential therapeutic application of DKK2 blockade in the clinical treatment of human CRC.
Keywords
Colorectal cancer, DKK2, Immunosuppression, Angiogenesis, Tumor microenvironment
The Targeted Therapies for CRC
CRC is one of the leading causes of cancer death in the United States, accounting for approximately 9% of all cancer deaths [1]. The major therapies for CRC include surgery resection, radiation, chemotherapies, and targeted therapies. The two major targeted therapies are Bevacizumab (Avastin) and Cetuximab (Erbitux), which targets VEGF and blood vessel formation, and epidermal growth factor receptor), respectively [1]. However, these anti-EGFR drugs do not work in CRC with mutations (defects) in the KRAS, NRAS, or BRAF gene [1]. Anti-VEGF agents have been widely used in the treatment of many solid cancers, including CRC [2,3]. However, the systemic administration of anti-VEGF monoclonal antibodies has been reported to cause tremendous adverse events including hypertension, impaired wound healing, hemorrhage and thrombosis, cardiac impairment, stroke, gastrointestinal perforations, and kidney disease [2,3]. These side effects reduce patient compliance and limit the application of anti-VEGF, particularly at the most effective dosage, on tumor therapy [2]. In colorectal cancer patients who were treated with bevacizumab combined with chemotherapy regimens, the median progression-free survival of metastatic colorectal cancer patients remained less than one year [2].
The Challenges for Immunotherapy in CRC
In recent years, immunotherapies have been devel¬oped to stimulate the capacity of immune effectors such as T cells to attack and eliminate tumor cells. Many immune therapies target inhibitory receptors or pathways that prevent or limit antitumor T cell responses and reactivate immune cells. Immune checkpoint blockade (ICB), including anti-PD1 and anti-CTLA4, is a strategy that directly tar¬gets T cells and re-stimulates them [4]. These immune-checkpoint inhibitors have shown clinical efficacy for some tumors, but not for many other tumors, which remain refractory or less susceptible to immune therapy [5,6]. This is the case in colorectal cancer. Most CRC belongs to the microsatellite stable (MSI-S) group, are refractory to the ICB treatment [7], despite occasionally exhibiting exceptionally good infiltration by immune cells. A major challenge in the field of immune therapy has thus become the understanding of primary and acquired resistance to ICB. Resistance to ICB may partly contribute to the redundancy of inhibitory receptors and pathways. Although mechanisms for resistance and/or insensitivity to current checkpoint inhibitors have been described [8], there are more mechanisms for tumor immune modulation yet to be discovered. Thus, we aimed to discover the new immunomodulators and investigate novel pathways in CRC.
DKKs in Tumor Immunosuppressive Microenvironment
Wnt signaling regulates many aspects of cellular processes. Its dysregulation causes developmental defects and diseases including cancer [9-11]. The Wnt/β-catenin signaling pathway is among the most studied. This pathway is deregulated in many tumors, particularly those lacking the gene adenomatous polypo¬sis coli (APC), a critical negative regulator of this pathway [12]. Wnt proteins bind to two types of cell-surface receptors: low-density lipoprotein receptor-related proteins 5 and 6 (LRP5 and LRP6, respectively), and Frizzled proteins, to initiate downstream pathways [9,13]. DKK1 and DKK2 are negative regulators of the Wnt pathway that interact with LRP5 and LRP6, preventing Wnt bind¬ing [9,13]. DKK1 was previously known to be secreted by latent metastatic cancer cells to downregulate ULBP ligands for NK cells and responsible for the evasiveness of NK-cell-mediated clearance [14]. Another study demonstrated that DKK1 regulates the accumulation and function of myeloid-derived suppressor cells in cancer [15].
The optimal candidates should be expressed at a lower level in various normal human tissues, especially in immune tissues. By this means, antibody blocking will not become a strong risk factor for autoimmunity. Likewise, the lack of these genes should not impede tissue development and cell function. DKK2 is one of these targets.
In our previous study published on Nature Medicine, we identified a novel inhibitory mechanism hampering natural killer (NK) and T cell function, involving the DKK2 protein, which confers resistance to ICB in colon cancer [16]. DKK2 was highly expressed in human colorectal cancer and, importantly, was correlated with individuals with poor survival [16]. The expression of DKK2 is regulated by the loss of APC [16]. To test the role of DKK2 in colorectal cancer, a monoclonal antibody against DKK2 was generated [17]. DKK2 blockade with the anti-DKK2 antibody was effective in suppressing tumor progression using a syngeneic CRC model grafted with mouse colon cancer cell MC38 and a genetic benign CRC model using the ApcMin/+ mice [16]. DKK2 secreted by tumor cells acts on cytotoxic lymphocytes, inhibiting STAT5 signaling by impeding STAT5 nuclear localization via LRP5, but independently of LRP6 and the Wnt–b-catenin pathway [16].
Mechanisms of DKK2 Antibody-Based Therapy for CRC
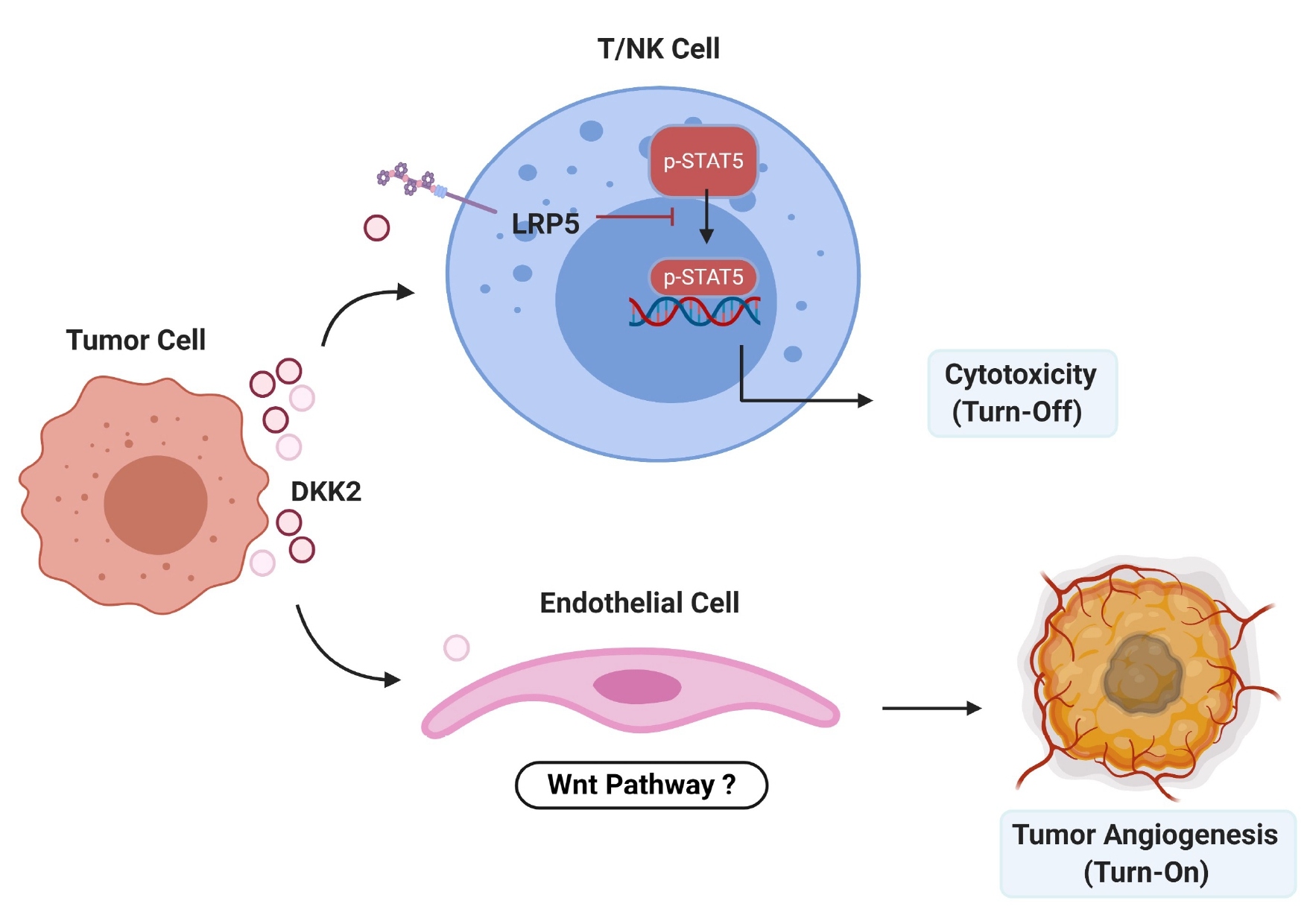
In our most recent studies, we generated a mouse genetic CRC model with mutations in APC and KRAS, which more closely mimics advanced human CRC, we confirmed the tumor inhibitory effect of DKK2 blockade, which significantly retarded tumor progression and extended survival, with increased immune effector cell activation and reduced angiogenesis [18]. We also established an ex vivo culture system to study the effect of DKK2 blockade on infiltrated cytotoxic immune cells from human CRC tumor samples. We demonstrated that the human colon tumors expressed high levels of DKK2, and DKK2 blockade caused stronger activation of tumor infiltrating CD8+ T cells. Intriguingly, we observed a correlation of high DKK2 expression with increased lymph node metastasis prevalence in these CRC patients as well [18]. Regarding to the clinical relevance, we found that the anti-DKK2 antibody synergizes with an anti-PD-1 antibody to promote the immune antitumor response in the ApcMin/+ mouse model, in part through the induction of GZMB on cytotoxic lympho¬cytes [16]. We have also expanded DKK2 blockers to treat lung cancer with mutations in APC and KRAS [19]. Previous studies demonstrated that DKK2 induction promoted angiogenesis in cultured human endothelial cells and in in vivo assays using mice [20,21]. In our study, we also reproduced the pro-angiogenic effects of recombinant DKK2 proteins [18]. On the other hand, Braumüller et al. demonstrated that IFNγ not only impedes tumor growth by acting directly on cancer cells [22], but also acted on the tumor stroma and tumor angiogenesis for effective rejection of large, established tumors [23-25]. Given that VEGF/VEGFR blockage had been approved to treat human CRC, we performed a combined administration of DKK2 blockade with sub-optimal anti-VEGFR treatment and observed a synergetic effect on suppressing tumor angiogenesis and progression, as well as extending survival, which yielded better results than those of every single therapy [18] (Figure 1).
Figure 1. Schematic summary of the proposed mechanism for the DKK2-mediated immunosuppressive pathway and angiogenesis for tumorigenesis. DKK2 secreted by tumor cells binds to LRP5 on NK and T cells, leading to sequestration of phosphorylated STAT5 to endosomes and reduction in its nuclear localization. This in turn leads to an impediment in NK/T cell activation including a reduction in granzyme B production and attenuated NK/T-mediated tumor cell killing. One the other hand, DKK2 directly promotes angiogenesis via regulating endothelial cells, this process might through the Wnt pathway.
Conclusions
Our research has identified previously unknown tumor immune-suppressive mechanism and immunotherapeutic targets specifically related to CRCs. The beneficial effect of the anti-DKK2 monoclonal antibody was not only in colorectal cancer models but also in mouse models of APC-deficient lung cancer [19], suggesting that DKK2 blockade may be used as a therapeutic approach alone or in combination with other ICB strategies in a wide range of tumors. Thus, our study provides further evidence for the therapeutic application of the DKK2 blockade in the clinical treatment of human CRC when combined with other targeted therapies.
References
2. Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. British Journal of Cancer. 2007 Jun;96(12):1788-95.
3. Niu G, Chen X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Current Drug Targets. 2010 Aug 1;11(8):1000-17.
4. Dyck L, Mills KH. Immune checkpoints and their inhibition in cancer and infectious diseases. European Journal of Immunology. 2017 May;47(5):765-79.
5. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015 Apr 13;27(4):450-61.
6. Daskivich TJ, Belldegrun A. Words of wisdom. Re: Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. European Urology. 2015 Apr 1;67(4):816-7.
7. Toh JW, de Souza P, Lim SH, Singh P, Chua W, Ng W, et al. The potential value of immunotherapy in colorectal cancers: review of the evidence for programmed death-1 inhibitor therapy. Clinical Colorectal Cancer. 2016 Dec 1;15(4):285-91.
8. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017 Feb 9;168(4):707-23.
9. Xiao Q, Chen Z, Jin X, Mao R, Chen Z. The many postures of noncanonical Wnt signaling in development and diseases. Biomedicine & Pharmacotherapy. 2017 Sep 1;93:359-69.
10. Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012 Jun 8;149(6):1192-205.
11. Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and β-catenin signalling: diseases and therapies. Nature Reviews Genetics. 2004 Sep;5(9):691-701.
12. Polakis P. Wnt signaling and cancer. Genes & Development. 2000 Aug 1;14(15):1837-51.
13. Niehrs C. The complex world of WNT receptor signalling. Nature Reviews Molecular Cell Biology. 2012 Dec;13(12):767-79.
14. Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell. 2016 Mar 24;165(1):45-60.
15. D’Amico L, Mahajan S, Capietto AH, Yang Z, Zamani A, Ricci B, et al. Dickkopf-related protein 1 (Dkk1) regulates the accumulation and function of myeloid derived suppressor cells in cancer. Journal of Experimental Medicine. 2016 May 2;213(5):827-40.
16. Xiao Q, Wu J, Wang WJ, Chen S, Zheng Y, Yu X, et al. DKK2 imparts tumor immunity evasion through β-catenin-independent suppression of cytotoxic immune-cell activation. Nature Medicine. 2018 Mar;24(3):262.
17. Zhao R, Xiao Q, Li M, Ren W, Xia C, Liu X, et al. Rational design of peptides for identification of linear epitopes and generation of neutralizing monoclonal antibodies against DKK2 for cancer therapy. Antibody Therapeutics. 2020 Apr;3(2):63-70.
18. Hu J, Wang Z, Chen Z, Li A, Sun J, Zheng M, et al. DKK2 blockage-mediated immunotherapy enhances anti-angiogenic therapy of Kras mutated colorectal cancer. Biomedicine & Pharmacotherapy. 2020 Jul 1;127:110229.
19. Shen T, Chen Z, Qiao J, Sun X, Xiao Q. Neutralizing monoclonal antibody against Dickkopf2 impairs lung cancer progression via activating NK cells. Cell Death Discovery. 2019 Jul 31;5(1):1-2.
20. Park H, Jung HY, Choi HJ, Kim DY, Yoo JY, Yun CO, et al. Distinct roles of DKK1 and DKK2 in tumor angiogenesis. Angiogenesis. 2014 Jan 1;17(1):221-34.
21. Min JK, Park H, Choi HJ, Kim Y, Pyun BJ, Agrawal V, et al. The WNT antagonist Dickkopf2 promotes angiogenesis in rodent and human endothelial cells. The Journal of Clinical Investigation. 2011 May 2;121(5):1882-93.
22. Braumüller H, Wieder T, Brenner E, Aßmann S, Hahn M, Alkhaled M, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013 Feb;494(7437):361-5.
23. Zhang B, Karrison T, Rowley DA, Schreiber H. IFN-γ–and TNF-dependent bystander eradication of antigen-loss variants in established mouse cancers. The Journal of Clinical Investigation. 2008 Apr 1;118(4):1398-404.
24. Hayakawa Y, Takeda K, Yagita H, Smyth MJ, Van Kaer L, Okumura K, et al. IFN-γ–mediated inhibition of tumor angiogenesis by natural killer T-cell ligand, α-galactosylceramide. Blood, The Journal of the American Society of Hematology. 2002 Sep 1;100(5):1728-33.
25. Kammertoens T, Friese C, Arina A, Idel C, Briesemeister D, Rothe M, et al. Tumour ischaemia by interferon-γ resembles physiological blood vessel regression. Nature. 2017 May;545(7652):98-102.