Abstract
Chemotherapy remains a cornerstone of modern cancer treatment, yet its neurotoxic side effects can pose significant challenges. Peripheral nerve damage and the resulting pain not only affect patients’ quality of life but also contribute to substantial economic burden. Despite the medical need, a definitive curative treatment for chemotherapy-induced peripheral neuropathy (CIPN) remains elusive, leaving many patients reliant on analgesics for pain relief. The QUCIP study, a prospective, multi-center observational study spanning 36 weeks, has recently yielded data evaluating the effectiveness of up to three treatments with high-concentration (179 mg) capsaicin patch (HCCP) in 73 female patients with painful CIPN following neoadjuvant/adjuvant breast cancer therapy. In this review, we discuss the results of the QUCIP trial and position its findings within the broader body of evidence on repeated HCCP treatment, highlighting its potential to progressively improve therapeutic outcomes and reduce the burden of CIPN in oncology care.
Keywords
Chemotherapy-induced peripheral neuropathy (CIPN), High-concentration capsaicin patch (HCCP), QUCIP study, Peripheral neuropathic pain, Topical treatment, Progressive response
Abbreviations:
ADR: Adverse Drug Reaction; ASCO: American Society of Clinical Oncology; CIPN: Chemotherapy-Induced Peripheral Neuropathy; EQ-5D-3L: European Quality of Life 5 Dimensions 3 Level; EQ-VAS: EuroQol-Visual Analogue Scales; ESMO: European Society for Medical Oncology; HCCP: High-Concentration Capsaicin Patch; NPRS: Numeric Pain Rating Scale; PDPN: Painful Diabetic Peripheral Neuropathy; PHN: Post-Herpetic Neuralgia; PNI: Post-operative or post-traumatic Nerve Injuries; QoL: Quality of Life; QLQ-CIPN20: Quality-of-Life Questionnaire-CIPN twenty-item scale; SD: Standard Deviation; TRPV1: Transient Receptor Potential Vanilloid 1; V: Visit
Introduction
The challenge of chemotherapy-induced peripheral neuropathy (CIPN)
Definition and causes: Neuropathic pain is defined as pain caused by a lesion or disease of the somatosensory (peripheral and/or central) nervous system, including loss of sensory function or sensation and increased pain sensitivity or spontaneous pain [1,2]. Chemotherapy-induced peripheral neuropathy (CIPN) is a common and sometimes dose-limiting side effect of oral or intravenous chemotherapy used to treat the primary tumor and/or its metastases [3,4]. Neurotoxic substances include platinum derivatives, taxanes (especially at high doses), vinca alkaloids, eribulin, bortezomib and thalidomide [5]. Furthermore, combinations of neurotoxic substances may have the potential to greatly increase peripheral neurotoxicity.
Neurotoxicity of chemotherapeutic agents affects various structures in the nervous system, including myelin sheaths, sensory cell bodies in the dorsal root ganglion, and axonal components (e.g., ion channels, microtubules, mitochondria). As a result, general degenerative processes and the production of pro-inflammatory cytokines as well as activation of apoptotic signaling cascades and alteration of neuronal excitability are initiated. Subsequently, these changes can lead to the loss of epidermal nerve fibers [6-8].
Clinical presentation: Nerve damage can affect sensory, motor, and autonomic nerves. Sensory and/or motor irritation and dysfunction usually predominate in feet, legs, arms, and hands. Peripheral neuropathic pain may be diffused or localized, the latter being present when the main pain can be localized to reproducible and circumscribed areas [9]. Sensory nerve fibers are particularly vulnerable and most susceptible to neurotoxic agents, which is why sensory symptoms are more prevalent. In CIPN, small (unmyelinated) as well as large (myelinated) peripheral nerve fibers can be affected. Symptoms of large fiber damage include numbness, impaired fine motor skills, reduced sensation of vibration and proprioception and a progressive loss of deep tendon reflexes, whereas symptoms of small fiber damage include burning pain and reduced nociceptive and thermal sensation [4].
Prevalence and impact: The incidence of CIPN mainly depends on the chemotherapeutic agents, the cumulative dose, and the duration of therapy. It is also influenced by prior therapies and the clinical setting [7]. A meta-analysis revealed a considerable range, from 12% to >95% across multiple types of cancer and chemotherapy regimens. The overall prevalence of CIPN is estimated to be 68% in the first month after the end of chemotherapy, 60% at three months, and 30% after ≥ 6 months (considered as chronic pain). This means that symptoms persist in about one third of patients after therapy cessation [10]. Depending on the substance administered (e.g., platinum compounds), further progression or re-emergence of symptoms even several months after therapy is possible (coasting phenomenon) [7,11,12].
The symptoms of CIPN can severely impact functionality and quality of life (QoL), disrupting many aspects of daily living. Affected individuals often face challenges such as sleep disorders, inability to work, gait instability, and balance disorders (tendency to stumble or fall), as well as difficulties with fine motor skills (impaired gripping and holding function of the hands, limitations when writing/working on the PC). If autonomic nerves are affected, symptoms may impact internal organs, manifesting as blood pressure instability, gastrointestinal disturbances, or impaired bladder function up to incontinence [5,13,14].
These limitations can lead to dose reductions or treatment discontinuation. Furthermore, substantial economic burden on the healthcare system is caused by long-term costs of untreated CIPN (e.g., increased care needs, loss of productivity [13,15].
Therapeutic options and the role of high-concentration capsaicin patch (HCCP)
Current treatment approaches: To date, no curative treatment options for CIPN are available. Therefore, CIPN management focuses on symptom control rather than reversal. International guidelines (e.g., American Society of Clinical Oncology [ASCO], European Society for Medical Oncology [ESMO]) prioritize duloxetine as first-line treatment for painful CIPN [11,16]. However, in addition to potential systemic side effects, the substance is not approved for the treatment of CIPN in all countries/regions [7]. Second-line options include gabapentinoids, tricyclic antidepressants, and serotonin-noradrenaline reuptake inhibitors (SNRIs), although data on their efficacy is limited, and it is important to consider the potential for systemic side effects as well as the labelling details when selecting an appropriate treatment [7]. Opioids may be used as well, but only as a third-line option [17,18].
Topical therapies, such as the high-concentration capsaicin patch (HCCP), are emerging alternatives as they are to be applied directly to the painful area where they exert their local mode of action. Consequently, most side effects are application site reactions which are transient and usually mild to moderate [7]. This treatment is particularly beneficial for elderly or multimorbid patients, including those with cancer, who are more susceptible to adverse effects. Moreover, patients express a preference for topical treatments, as local side effects are more likely to be accepted than systemic side effects [19].
HCCP’s position in neuropathic pain guidelines: According to neuropathic guidelines, HCCP is a second-line option [18,20] and suitable for localized neuropathic pain as an early or first-line treatment choice [20].
Evidence Supporting HCCP in Peripheral Neuropathic Pain
Approval and mode of action
HCCP has been granted approval for the treatment of neuropathic pain of any origin in the European Union, as well as for post-herpetic neuralgia (PHN) and painful diabetic peripheral neuropathy (PDPN) in the United States [21,22]. Treatment can be repeated every 90 days, as warranted by persistence or return of pain. However, retreatment after a minimum interval of 60 days can be considered for individual patients after physician assessment [23].
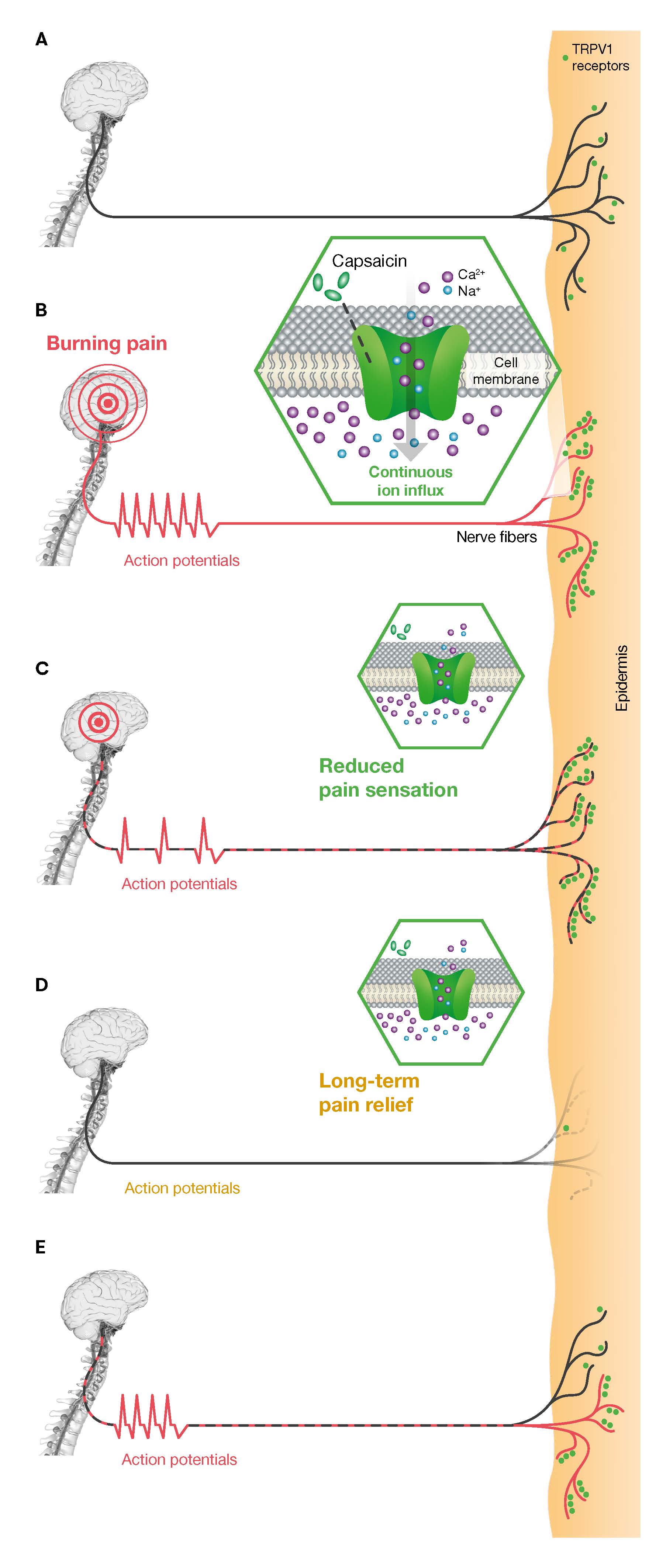
The active ingredient of HCCP, capsaicin, specifically targets the transient receptor potential vanilloid 1 (TRPV1) receptor, which is overexpressed in peripheral neuropathic pain, contributing to nerve fiber hyperactivity. Binding of capsaicin to TRPV1 triggers action potentials and the release of vasoactive neuropeptides, which may cause transient sensations of burning pain, stinging, and erythema. This is followed by a reduction in TRPV1 receptor sensitivity to various stimuli, providing temporary pain relief. Prolonged pain reduction is finally achieved through the reversible defunctionalization of epidermal nociceptors, lasting three to five months [23-25]. This mechanism selectively targets TRPV1-expressing nociceptors without compromising other sensory modalities, such as cold or touch perception [26]. Interestingly, in patients with CIPN and other neuropathic pain conditions, nerve biopsies have shown the regrowth of intraepidermal and subepidermal nerve fibers after treatment, which appears to correlate with pain reduction [27-31]. These observations suggest that following the reversible defunctionalization of affected nerve fibers by capsaicin, some fibers regenerate with hypersensitivity, while others recover with restored normal function, indicating a potential for disease-modifying effects (Figure 1).
Figure 1. Mode of action of HCCP and potential mechanism of disease modification. (A) Healthy condition: In a healthy state, nerve fiber density and the expression of TRPV1 receptors (green dots) in the epidermis remain at normal levels. (B) Sensitization of the TRPV1 receptor (burning pain): Neuropathic pain is caused by reduced nerve fiber density and disease-related overexpression of excitatory ion channels, including the TRPV1 receptor. Capsaicin activates the TRPV1 receptor, causing the ion channel to open. This leads to an influx of calcium and sodium ions, triggering a cascade of action potentials that patients often perceive as a burning pain. (C) Desensitization of the TRPV1 receptor (pain reduction): Continuous activation with high concentrations of capsaicin leads to temporary desensitisation of the TRPV1 receptor, reducing the perception of pain. (D) Reversible defunctionalization of the nerve fibers (long-term pain relief): The increase in intracellular calcium concentration triggers cellular damage, leading to the reversible defunctionalization of nerve fibers, providing sustained pain relief. (E) Nerve regeneration: Nerve fibers typically regenerate within three months following capsaicin administration. While some may regain pathological hypersensitivity, necessitating repeated HCCP treatments, others may regenerate with normal sensitivity, indicating a potential for disease modification. Modified from [25,27-29,31]. HCCP: High-Concentration Capsaicin Patch; TRPV1: Transient Receptor Potential Vanilloid 1.
Clinical evidence
The efficacy of HCCP has been demonstrated in a range of conditions, including PHN, PDPN, HIV-associated neuropathic pain and neuropathic pain resulting from post-operative or post-traumatic nerve injuries (PNI) [28,32-42]. In addition, several studies have indicated that HCCP may also be an effective treatment for CIPN [7,27,43-46].
HCCP in CIPN: Insights from the QUCIP Study and Beyond
Study overview
QUCIP was a prospective, multicenter, observational study to assess the effectiveness and safety of HCCP over 36 weeks with up to three HCCP treatments in breast cancer patients with CIPN. The focus of the study was to evaluate pain intensity, impact on neuropathic symptoms, QoL, and tolerability of the treatment [47]. The painDETECTÒ questionnaire was used to identify the neuropathic pain phenotype in patients. It employs a scoring system to assess the probability of neuropathic pain, with higher scores suggesting a greater likelihood of neuropathic pain components. The questionnaire comprises seven individually weighted (0-5 scale) sensory symptoms of neuropathic pain, as well as two items pertaining to pain characteristics. A final score of ≥ 19 is indicative of a high likelihood of neuropathic pain, whereas a score of ≤ 12 suggests the presence of predominantly nociceptive pain [48,49]. The painDETECTÒ questionnaire has also been shown to be a valuable tool for assessing pain severity and monitoring changes in the intensity of sensory symptoms in patients with neuropathic pain throughout the course of treatment [48,50].
Key findings
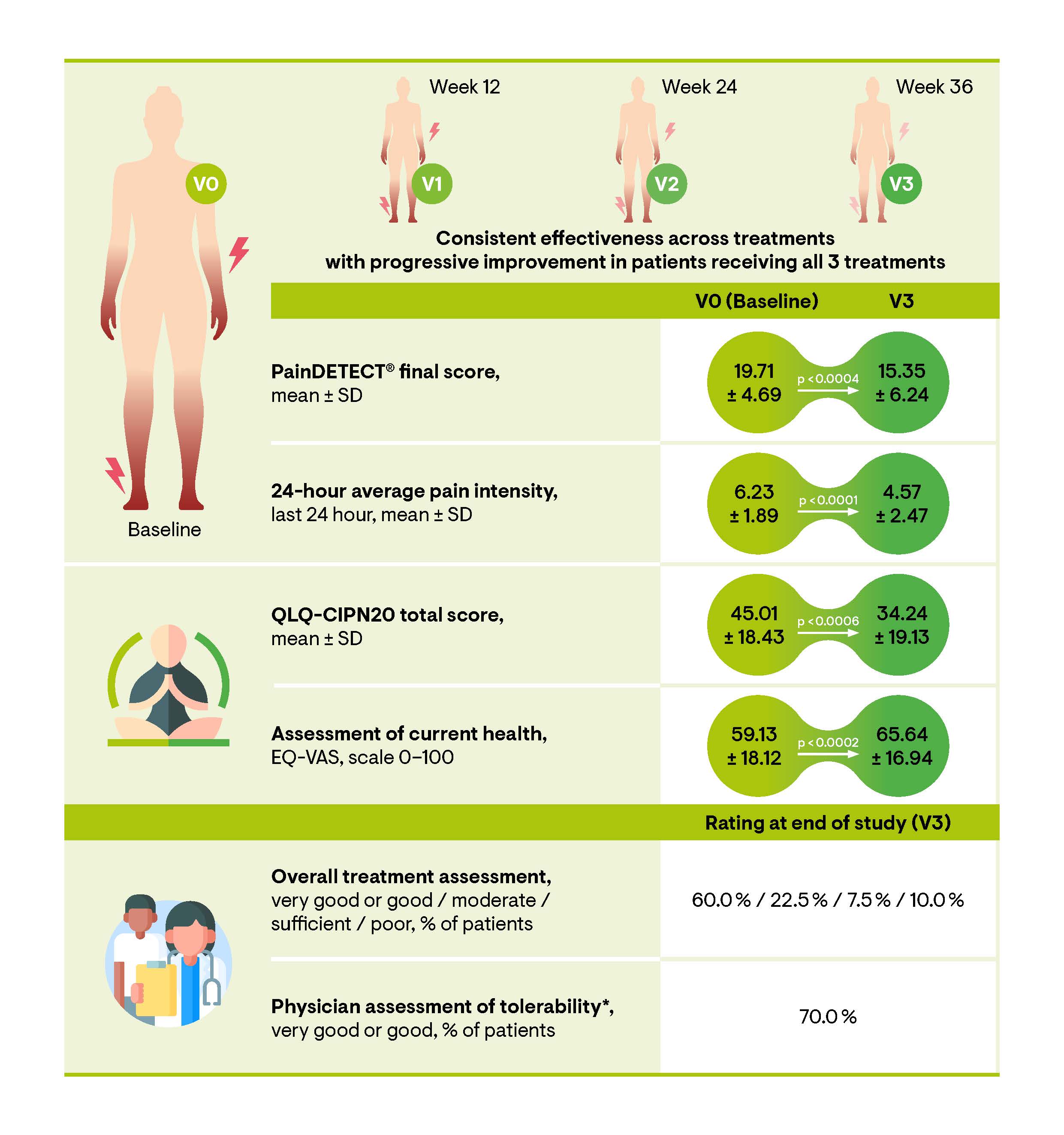
The QUCIP study evaluated data from 73 patients who received at least one treatment with HCCP. The median age of the study cohort at baseline was 61 years, with an age range of 36–80 years. Over half of the patients (52.0%) had experienced peripheral neuropathic pain for more than one year, with 34.2% reporting symptoms persisting beyond two years. The study demonstrated a substantial reduction in neuropathic pain symptomatology, reflected by a significant decrease in neuropathic pain scores according to the painDETECTÒ questionnaire. The painDETECTÒ final score decreased from 19.71 (±4.69) at baseline to 15.80 (±6.20) following the initial HCCP treatment (p<0.0001) at visit 1 (V1). This reduction was further observed at subsequent follow-up visits 2 (V2) and 3 (V3). By V3, patients who received three consecutive HCCP treatments achieved a painDETECTÒ final score below 12, suggesting that HCCP treatment is effective in alleviating the clinical manifestations of neuropathic pain. Furthermore, a significant reduction in the numeric pain rating scale (NPRS) score was observed after the initial treatment (p<0.0001) (Figure 2). Both scores exhibited cumulative benefits following repeated treatments. In addition, the proportions of patients achieving a clinically significant pain relief of ≥ 30% or ≥ 50% increased from 25.0% and 14.7% at the 4-week follow-up to 40.0% and 32.5% at week 36 (V3), respectively [47].
Patients exhibited an improvement in neuropathic symptoms, as shown by enhanced sensory function over time. The painDETECTÒ questionnaire revealed a significant reduction in the presence and severity of the seven neuropathic symptoms (i.e., burning sensation, tingling sensation, allodynia, pain attacks, thermal hyperalgesia, numbness, pressure-evoked pain) from baseline to V3 and these improvements remained stable until the end of the study visit (V3) (p<0.0001 for each visit compared to baseline). At baseline, the most prominent symptoms were tingling and numbness, followed by burning sensation, pain attacks, and thermal hyperalgesia. Significant improvements were observed at V1 for tingling, allodynia, pain attacks and numbness. By V2 and/or V3, all neuropathic symptoms except pressure-evoked pain showed significant improvement. The significant reduction of numbness and tingling, primary non-painful sensations, along with the reduction of the painDETECTÒ final score to below 12 in patients receiving up to three treatments, may support the hypothesis of a potential disease-modifying effect [27]. In addition, the QUCIP study revealed marked improvements in QoL, as measured by the Quality-of-Life Questionnaire-CIPN twenty-item scale (QLQ-CIPN20) and European Quality of Life 5 Dimensions 3 Level (EQ-5D-3L) scores. Patient evaluations further support the effectiveness of HCCP treatment, with 60% rating it as very good or good and 22.5% describing it as moderate (Figure 2) [47]. The most reported adverse drug reactions (ADRs) were application site pain (24.7%), burning sensation (8.2%), and application site erythema (4.1%). Overall, the treatment was well-tolerated, with predominantly mild to moderate local side effects consistently observed across single and multiple HCCP treatments, as demonstrated in other investigations [7,43,44,47,51,52].
Figure 2. Sustained effectiveness across HCCP treatments, with progressive improvement following each additional treatment (n=73). In the QUCIP trial, the effects of up to three HCCP treatments, administered every 12 weeks over a 36-week period, were monitored. Following the initial HCCP treatment (V0), telephone follow-ups were conducted at 2 days and 4 weeks. Face-to-face visits occurred every 12 weeks and/or at the time of retreatment (V1–V3). Modified from [47]. *85% of patients remaining in the study until EoS reported not experiencing side effects from the treatment. The presented data are based on an observed cases (OC) analysis. EQ-VAS: EuroQol-Visual Analogue Scales; HCCP: High-Concentration Capsaicin Patch; QLQ-CIPN20: Quality of Life Questionnaire-CIPN twenty-item scale; SD: Standard Deviation; V: Visit
Supporting evidence from other studies
HCCP treatment has been shown to be effective in several other study settings. A post-hoc analysis of the PACE and STRIDE trials, involving repeated treatments over 12 months, demonstrated a progressive response to HCCP, with even initial non-responders benefiting from subsequent treatments [51]. Furthermore, Kern et al. demonstrated that retreatment with HCCP was more effective and resulted in a notable reduction in opioid use [52]. Further retrospective studies have highlighted the efficacy of HCCP treatment in a subset of patients experiencing neuropathic pain following breast cancer therapy, demonstrating enhanced pain relief and improved QoL [43,44]. These data also indicate higher response rates after early use in the treatment pathway. All studies confirmed the favorable safety profile for long-term use with localized and transient side effects.
The level of response to the HCCP treatment may vary depending on the specific chemotherapeutic agent that was applied. For example, efficacy appears to be lower for platinum salts-induced CIPN compared to other chemotherapies [43]. This may be due to differences in how chemotherapies affect TRPV1 pathways, potentially leading to distinct types of CIPN that vary in their response to TRPV1 agonist treatment. However, the underlying mechanisms remain poorly understood. A better understanding of the specific pathophysiological processes that drive CIPN in different chemotherapies and cancer types could lead to the development of more targeted and effective treatments. While most studies on neuropathic pain emphasize the early initiation of HCCP treatment to optimize outcomes [44,50,53], the QUCIP study demonstrated a consistent reduction in pain intensity across all groups, regardless of pain duration [47]. Conversely, a cohort of CIPN patients from a comprehensive chart review on peripheral neuropathic pain following breast cancer treatment showed that those with pain lasting over two years responded significantly better to treatment than those with shorter pain durations [43]. These findings suggest that pain duration may have less impact on treatment effectiveness in CIPN compared to other forms of peripheral neuropathic pain. However, due to the limited size of the pain duration subgroups, definitive conclusions cannot be drawn.
Summary & Conclusion
Summary of evidence
The data from the QUCIP study in CIPN patients are in line with the current literature on the treatment of neuropathic pain using HCCP, including consistent reductions in neuropathic symptoms, improved pain relief, and enhanced QoL [28,43,44,47,51,52,54-56]. Furthermore, repeated HCCP applications deliver cumulative and long-term benefits [43,44,47,51]. It is therefore crucial that patients are adequately informed that repeated treatments may be necessary for successful therapy. In line with this, the European Medicines Agency recommends that treatment effectiveness should be assessed after three cycles to determine whether continuation is appropriate [23].
The favorable tolerability profile of HCCP treatment, which is characterized by predominantly localized and transient side effects, is particularly relevant in the context of cancer-related neuropathic pain, where systemic treatments can carry significant risks, and the affected patient population is often elderly and presents with multiple comorbidities.
Clinical implications
Given the high individual and socioeconomic burden associated with CIPN [13], early intervention and retreatment are crucial to optimize therapeutic outcomes and improve patient QoL. This is supported by the majority of QUCIP physicians, rating the integration of the HCCP therapy into clinical routine as very good or good [47]. The application can be readily integrated into existing procedures and, following initial training, is suitable for use in private practice settings.
Early consideration of HCCP in future CIPN management guidelines will help to ensure that patients receive the maximum benefit from this therapeutic option. However, further research is needed to better understand the full potential of capsaicin in CIPN, i.e., with respect to the dosage and/or type of specific cancer treatments and underlying pathophysiological processes.
Competing Interests
RS: Grünenthal GmbH. M.P.L.: Participation on advisory boards for AstraZeneca, Lilly, MSD, Novartis, Hexal, Pfizer, Eisai, Gilead, Exact Sciences, Daiichi-Sankyo, and Roche and has received honoraria for lectures from MSD, Lilly, Roche, Novartis, Hexal, Pfizer, Exact Sciences, Daiichi-Sankyo, Grünenthal, Gilead, AstraZeneca, and Eisai. He received travel expenses from Gilead, Pfizer and Daiichi-Sankyo. TQ: employed by Grünenthal GmbH.
Funding
Editorial assistance for the writing of this article was provided by Carmen Koch-Stork, PhD (KW medipoint) funded by Grünenthal GmbH.
Author Contributions
All authors were involved in the conception, design, and drafting of the manuscript. Medical writing support was provided under the authors' guidance. All authors have reviewed and approved the final manuscript.
References
2. Scholz J, Finnerup NB, Attal N, Aziz Q, Baron R, Bennett MI, et al. Classification Committee of the Neuropathic Pain Special Interest Group (NeuPSIG). The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. 2019 Jan;160(1):53-9.
3. Gießen-Jung C, von Baumgarten L. Chemotherapie-induzierte periphere Neuropathie. DMW- Dtsch Med Wochenschr. 2018 Jul;113(13):970-8.
4. Naleschinski D, Baron R, Miaskowski C. Identification and treatment of neuropathic pain in patients with cancer. Pain Clin Updates. 2012;20(2):1-4.
5. S3-Leitlinie Supportive Therapie, Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Supportive Therapie bei onkologischen PatientInnen - Langversion 1.3, Februar 2020, AWMF Registriernummer 032/054OL. Abgerufen am: 19.06.2020; https://www.leitlinienprogramm-onkologie.de/leitlinien/supportive-therapie/ 2020.
6. Addington J, Freimer M. Chemotherapy-induced peripheral neuropathy: an update on the current understanding. F1000Res. 2016 Jun 22;5:F1000 Faculty Rev-1466.
7. Maihöfner C, Diel I, Tesch H, Quandel T, Baron R. Chemotherapy-induced peripheral neuropathy (CIPN): current therapies and topical treatment option with high-concentration capsaicin. Support Care Cancer. 2021 Aug;29(8):4223-38.
8. Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev. 2014 Aug;40(7):872-82.
9. Mick G, Baron R, Finnerup NB, Hans G, Kern KU, Brett B, et al. What is localized neuropathic pain? A first proposal to characterize and define a widely used term. Pain Manag. 2012 Jan;2(1):71-7.
10. Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014 Dec;155(12):2461-70.
11. Loprinzi CL, Lacchetti C, Bleeker J, Cavaletti G, Chauhan C, Hertz DL, et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J Clin Oncol. 2020 Oct 1;38(28):3325-48.
12. Zajączkowska R, Kocot-Kępska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int J Mol Sci. 2019 Mar 22;20(6):1451.
13. Kerckhove N, Collin A, Condé S, Chaleteix C, Pezet D, Balayssac D. Long-Term Effects, Pathophysiological Mechanisms, and Risk Factors of Chemotherapy-Induced Peripheral Neuropathies: A Comprehensive Literature Review. Front Pharmacol. 2017 Feb 24;8:86.
14. Mols F, Beijers T, Vreugdenhil G, van de Poll-Franse L. Chemotherapy-induced peripheral neuropathy and its association with quality of life: a systematic review. Support Care Cancer. 2014 Aug;22(8):2261-9.
15. Pike CT, Birnbaum HG, Muehlenbein CE, Pohl GM, Natale RB. Healthcare costs and workloss burden of patients with chemotherapy-associated peripheral neuropathy in breast, ovarian, head and neck, and nonsmall cell lung cancer. Chemother Res Pract. 2012;2012:913848.
16. Jordan B, Margulies A, Cardoso F, Cavaletti G, Haugnes HS, Jahn P, et al. ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org; EONS Education Working Group. Electronic address: eons.secretariat@cancernurse.eu; EANO Guideline Committee. Electronic address: office@eano.eu. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO-EONS-EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann Oncol. 2020 Oct;31(10):1306-19.
17. Attal N, Cruccu G, Baron R, Haanpää M, Hansson P, Jensen TS, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010 Sep;17(9):1113-e88.
18. Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015 Feb;14(2):162-73.
19. Schubert T, Kern KU, Schneider S, Baron R. Oral or Topical Pain Therapy-How Would Patients Decide? A Discrete Choice Experiment in Patients with Peripheral Neuropathic Pain. Pain Pract. 2021 Jun;21(5):536-46.
20. Schlereth, T. and et al., Diagnosis and non-interventional therapy of neuropathic pain [Diagnose und nicht interventionelle Therapie neuropathischer Schmerzen]; S2k-level guideline of the Deutsche Gesellschaft für Neurologie; Guidelines for Diagnostics and Therapy in Neurology. Access date: 11/13/2019; https://www.dgn.org/leitlinien. 2019.
21. EMA, European Medicines Agency (EMA). https://www.ema.europa.eu/en/medicines/human/EPAR/qutenza#authorisation-details-section. Accessed 31. Aug. 2023. 2023.
22. FDA, U. S. Food and Rug Administration; Approval Letter Qutenza; accessed 07/04/2024 via: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022395s000Approv.pdf. 2010.
23. SmPC, European Medicines Agency (EMA). Qutenza 179 mg cutaneous patch: EU summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/qutenza-epar-product-information_en.pdf. Accessed 05 Dec. 2024. 2024.
24. Mou J, Paillard F, Turnbull B, Trudeau J, Stoker M, Katz NP. Qutenza (capsaicin) 8% patch onset and duration of response and effects of multiple treatments in neuropathic pain patients. Clin J Pain. 2014 Apr;30(4):286-94.
25. Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth. 2011 Oct;107(4):490-502.
26. Lo Vecchio S, Andersen HH, Arendt-Nielsen L. The time course of brief and prolonged topical 8% capsaicin-induced desensitization in healthy volunteers evaluated by quantitative sensory testing and vasomotor imaging. Exp Brain Res. 2018 Aug;236(8):2231-44.
27. Anand P, Elsafa E, Privitera R, Naidoo K, Yiangou Y, Donatien P, et al. Rational treatment of chemotherapy-induced peripheral neuropathy with capsaicin 8% patch: from pain relief towards disease modification. J Pain Res. 2019 Jul 3;12:2039-52.
28. Anand P, Privitera R, Donatien P, Fadavi H, Tesfaye S, Bravis V, et al. Reversing painful and non-painful diabetic neuropathy with the capsaicin 8% patch: Clinical evidence for pain relief and restoration of function via nerve fiber regeneration. Front Neurol. 2022 Oct 26;13:998904.
29. Anand P, Privitera R, Donatien P, Misra VP, Woods DR. Capsaicin 8% Patch Treatment in Non-Freezing Cold Injury: Evidence for Pain Relief and Nerve Regeneration. Front Neurol. 2021 Aug 19;12:722875.
30. Curatolo M. Pain relief after topical capsaicin: does it result from nociceptor degeneration or regeneration? Pain. 2023 Mar 1;164(3):461-2.
31. Sendel M, Dunst A, Forstenpointner J, Hüllemann P, Baron R. Capsaicin treatment in neuropathic pain: axon reflex vasodilatation after 4 weeks correlates with pain reduction. Pain. 2023 Mar 1;164(3):534-42.
32. Backonja M, Wallace MS, Blonsky ER, Cutler BJ, Malan P Jr, Rauck R, et al. NGX-4010 C116 Study Group. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomised, double-blind study. Lancet Neurol. 2008 Dec;7(12):1106-12.
33. Backonja MM, Malan TP, Vanhove GF, Tobias JK; C102/106 Study Group. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomized, double-blind, controlled study with an open-label extension. Pain Med. 2010 Apr;11(4):600-8.
34. Clifford DB, Simpson DM, Brown S, Moyle G, Brew BJ, Conway B, et al. NGX-4010 C119 Study Group. A randomized, double-blind, controlled study of NGX-4010, a capsaicin 8% dermal patch, for the treatment of painful HIV-associated distal sensory polyneuropathy. J Acquir Immune Defic Syndr. 2012 Feb 1;59(2):126-33.
35. Haanpää M, Cruccu G, Nurmikko TJ, McBride WT, Docu Axelarad A, Bosilkov A, et al. Capsaicin 8% patch versus oral pregabalin in patients with peripheral neuropathic pain. Eur J Pain. 2016 Feb;20(2):316-28.
36. Irving GA, Backonja MM, Dunteman E, Blonsky ER, Vanhove GF, Lu SP, et al. NGX-4010 C117 Study Group. A multicenter, randomized, double-blind, controlled study of NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia. Pain Med. 2011 Jan;12(1):99-109.
37. Simpson DM, Brown S, Tobias J; NGX-4010 C107 Study Group. Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology. 2008 Jun 10;70(24):2305-13.
38. Simpson DM, Robinson-Papp J, Van J, Stoker M, Jacobs H, Snijder RJ, et al. Capsaicin 8% Patch in Painful Diabetic Peripheral Neuropathy: A Randomized, Double-Blind, Placebo-Controlled Study. J Pain. 2017 Jan;18(1):42-53.
39. Vinik AI, Perrot S, Vinik EJ, Pazdera L, Jacobs H, Stoker M, et al. Capsaicin 8% patch repeat treatment plus standard of care (SOC) versus SOC alone in painful diabetic peripheral neuropathy: a randomised, 52-week, open-label, safety study. BMC Neurol. 2016 Dec 6;16(1):251.
40. Vinik AI, Perrot S, Vinik E, Pazdera L, Stoker M, Snijder R, et al. Repeat treatment with capsaicin 8% patch (179mg capsaicin cutaneous patch): effects on pain, quality of life, and patient satisfaction in painful diabetic peripheral neuropathy: an open-label, randomized controlled clinical trial. Journal of Current Medical Research and Opinion. 2019 Dec 24;2(12):388-401.
41. Webster LR, Malan TP, Tuchman MM, Mollen MD, Tobias JK, Vanhove GF. A multicenter, randomized, double-blind, controlled dose finding study of NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia. J Pain. 2010 Oct;11(10):972-82.
42. Webster LR, Tark M, Rauck R, Tobias JK, Vanhove GF. Effect of duration of postherpetic neuralgia on efficacy analyses in a multicenter, randomized, controlled study of NGX-4010, an 8% capsaicin patch evaluated for the treatment of postherpetic neuralgia. BMC Neurol. 2010 Oct 11;10:92.
43. Bienfait F, Julienne A, Jubier-Hamon S, Seegers V, Delorme T, Jaoul V, et al. Evaluation of 8% Capsaicin Patches in Chemotherapy-Induced Peripheral Neuropathy: A Retrospective Study in a Comprehensive Cancer Center. Cancers (Basel). 2023 Jan 5;15(2):349.
44. Dupoiron D, Jubier-Hamon S, Seegers V, Bienfait F, Pluchon YM, Lebrec N, et al. Peripheral Neuropathic Pain Following Breast Cancer: Effectiveness and Tolerability of High-Concentration Capsaicin Patch. J Pain Res. 2022 Feb 1;15:241-55.
45. Filipczak-Bryniarska I, Krzyzewski RM, Kucharz J, Michalowska-Kaczmarczyk A, Kleja J, Woron J, et al. High-dose 8% capsaicin patch in treatment of chemotherapy-induced peripheral neuropathy: single-center experience. Med Oncol. 2017 Aug 17;34(9):162.
46. Maihofner C, Heskamp ML. Prospective, non-interventional study on the tolerability and analgesic effectiveness over 12 weeks after a single application of capsaicin 8% cutaneous patch in 1044 patients with peripheral neuropathic pain: first results of the QUEPP study. Curr Med Res Opin. 2013 Jun;29(6):673-83.
47. Lux MP, Flöther L, Frömter C, Rack B, Veselinovic K, Heine M, et al. Topical treatment of chemotherapy-induced peripheral neuropathy (CIPN) with high-concentration (179 mg) capsaicin patch in breast cancer patients - results of the QUCIP study. Front Oncol. 2024 Sep 6;14:1452099.
48. Cappelleri JC, Bienen EJ, Koduru V, Sadosky A. Measurement properties of painDETECT by average pain severity. Clinicoecon Outcomes Res. 2014 Nov 6;6:497-504.
49. Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006 Oct;22(10):1911-20.
50. Höper J, Helfert S, Heskamp ML, Maihöfner CG, Baron R. High concentration capsaicin for treatment of peripheral neuropathic pain: effect on somatosensory symptoms and identification of treatment responders. Curr Med Res Opin. 2014 Apr;30(4):565-74.
51. Freynhagen R, Argoff C, Eerdekens M, Engelen S, Perrot S. Progressive Response to Repeat Application of Capsaicin 179 mg (8% w/w) Cutaneous Patch in Peripheral Neuropathic Pain: Comprehensive New Analysis and Clinical Implications. Pain Med. 2021 Oct 8;22(10):2324-36.
52. Kern KU, Quandel T, Theis S, Schubert T. Characteristics and outcomes of peripheral neuropathic pain patients with repeated applications of high-concentration capsaicin cutaneous patch: Results of a retrospective chart review in Germany. Pain Pract. 2024 Jun;24(5):700-8.
53. Maihöfner CG, Heskamp ML. Treatment of peripheral neuropathic pain by topical capsaicin: Impact of pre-existing pain in the QUEPP-study. Eur J Pain. 2014 May;18(5):671-9.
54. Mullins CF, Walsh S, Rooney A, McCrory C, Das B. A preliminary prospective observational study of the effectiveness of high-concentration capsaicin cutaneous patch in the management of chronic post-surgical neuropathic pain. Ir J Med Sci. 2022 Apr;191(2):859-64.
55. Santos MP, Lemos F, Gomes J, Romão JM, Veiga D. Topical capsaicin 8% patch in peripheral neuropathic pain: Efficacy and quality of life. Br J Pain. 2024 Feb;18(1):42-56.
56. Vieira IF, de Castro AM, Loureiro MDC, Pinto J, Cardoso C, Assuncao JP. Capsaicin 8% for Peripheral Neuropathic Pain Treatment: A Retrospective Cohort Study. Pain Physician. 2022 Jul;25(4):E641-7.