Commentary
In a recent attempt to elucidate the role of HIF1α in modulating the fate of T cells [1], we have demonstrated that the targeted deletion of HIF1α, specifically in T cells, enhances the inflammatory capabilities and memory phenotype of CD8+ T cells. These findings validate that the T cell-specific ablation of HIF1α or pharmacological inhibition of HIF1α reduces the development of B16F10 melanomas, suggesting an improved anti-tumor immune response characterized by improved secretion of effector molecules, such as IFN-γ, granzyme B, and TNFα, in the CD8+ T cells [1]. Thus, it appears that HIF1α might negatively influence specific effector functions of CD8+ T cells, which are crucial for destroying and targeting tumor cells (Figure 1). Consequently, the inhibition of HIF1α could lead to a multifaceted enhancement of anti-tumor immunity.
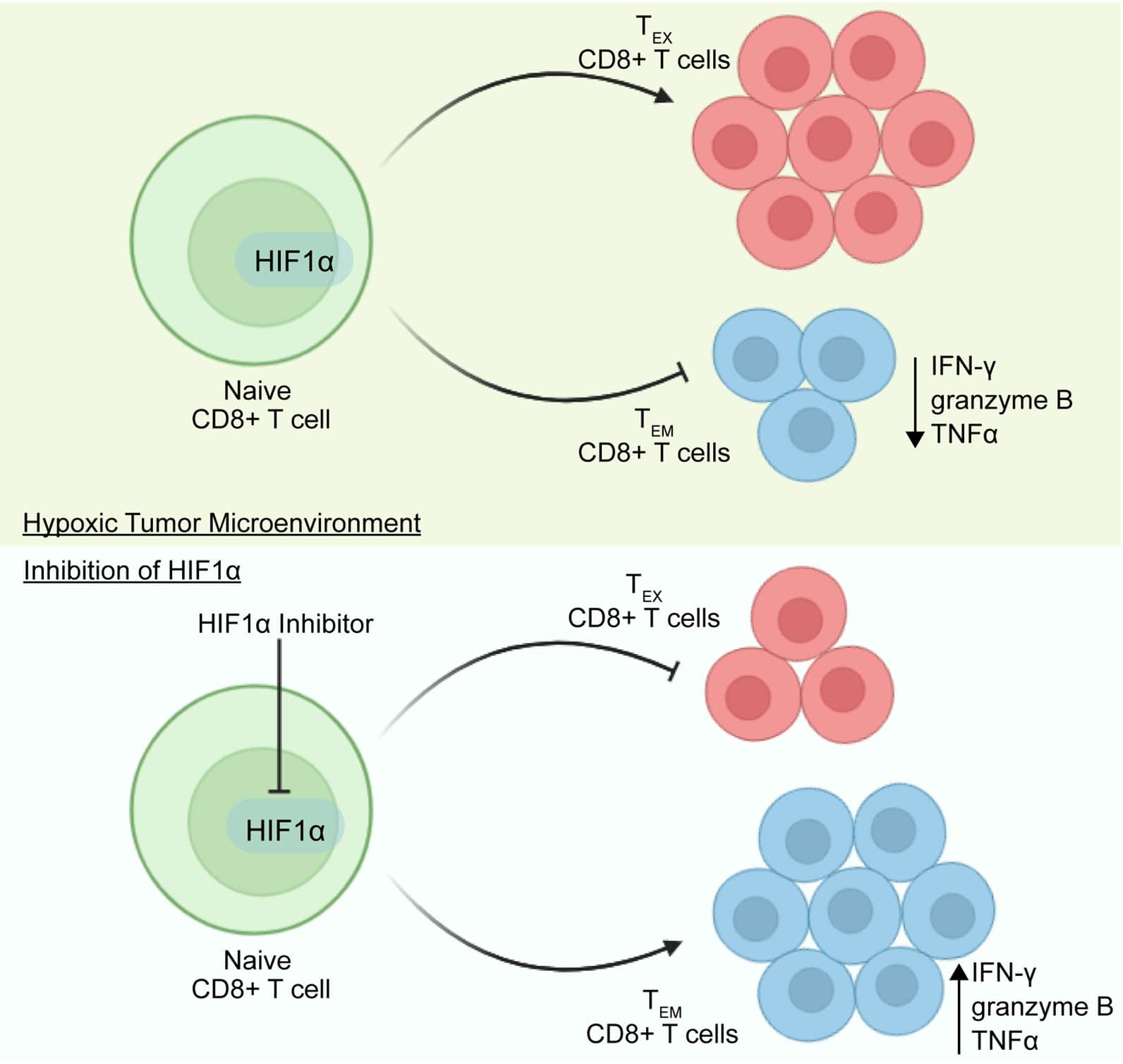
Figure 1. Blocking HIF1α in T cells enhances effector-memory function in CD8+ T cells to bolster anti-tumor immunity. Under hypoxic conditions in the tumor microenvironment, activation of HIF1α leads to the exhaustion of CD8+ T cells, along with a decrease in the effector CD8+ T cells. However, pharmacological or genetic inhibition of HIF1α improves the population of CD8+ TEM cells and enhances the secretion of effector molecules from CD8+ T cells.
CD8+ T cells are pivotal constituent of the adaptive immune system in the fight against cancers. Upon activation, naïve CD8+ T cells differentiate towards effector and memory CD8+ T cells [2]. The differentiation of CD8+ T cells is highly dependent on the transcriptional, epigenetic, and metabolic reprogramming events [3]. Besides, factors influencing the tumor microenvironment, such as hypoxia, also influence the differentiation of CD8+ T cells [4]. While hypoxia is a prevalent metabolic stress found in tumor microenvironments, terminally exhausted intratumoral T cells appear to experience it to a greater extent, indicating that hypoxia may play a significant role in CD8+ T cell biology [5]. Mechanistically, under normoxic (normal oxygen) conditions, prolyl-hydroxylases (PHDs) play a critical role by assisting the hypoxia-inducible factor-1 alpha (HIF1α) subunits in hydroxylating at specific sites where two conserved prolines are located. This hydroxylation process stabilizes the HIF1α subunits. Subsequently, the Von Hippel–Lindau (VHL) protein takes the lead in orchestrating the ultimate proteasomal degradation of HIF1α through a process known as ubiquitination and acts as a negative regulator of HIF1α [6,7].
Despite the well-established association between the hypoxic microenvironment in inflamed tissues and tumors and its role in immunosuppression and T-cell differentiation [8-10], some researchers have provided solid evidence for the notion that hypoxic conditions, along with the activity of HIF1α, can augment the differentiation of CD8+ T cells into cytotoxic T cells with improved effector function more effectively [11,12]. These findings have been validated in mice with genetic ablation of VHL in the T cells, which elevated the expression of HIFs, including HIF1α and HIF2α. For example, in a study, mice developed with VHL-deficient CD8+ T cells exhibited upregulated HIF1α and HIF2α and controlled B16 tumor development [12]. Meanwhile, VHL-deficient CD8+ T cells also induce glycolytic metabolism and enhance the effector functions in CD8+ T cells [12]. Similarly, another contrasting study used HIF1αfl/fllckCRE mice and exhibited elevated tumor growth and depleted effector differentiation of CD8+ T cells [13]. Additionally, the deletion of VHL in CD8+ T cells promoted the differentiation of memory CD8+ T cells in a manner dependent on both HIF1α and HIF2α. Interestingly, the heightened anti-tumor potency observed in CD8+ T cells was primarily attributed to the indirect but constitutive activity of HIF1α [14]. The primary limitation of these studies lies in their reliance on mouse models where molecules associated with HIF1α signaling were conditionally knocked out instead of direct conditional knockout of HIF1α in T cells. This approach may not fully capture the specific and elusive effects of HIF1α within T cells, potentially leading to incomplete or indirect insights into its role in T cell biology. To address these limitations, authors have taken the step of generating T-cell-specific HIF1α knockout mice. Surprisingly, our findings revealed an enhanced anti-tumor immune response characterized by heightened effector functions in CD8+ T cells. To further validate these findings, tumor-bearing mice and purified T cells were treated with a HIF1α inhibitor, Acriflavine (ACF). The results mirrored those obtained with HIF1α ablation, manifesting a decline in tumor growth and an enhancement in the memory and inflammatory potential of CD8+ T cells [1]. Besides our findings, which exhibited that inhibiting the HIF1α using ACF enhances anti-tumor immune responses, it has also been shown that various other inhibitors of HIF1α, such as dichloroacetate and PX-478, exhibit anti-tumor immune responses [15,16]. This unexpected result highlights the significance of HIF1α depletion in achieving anti-tumor immune responses.
Upon activation of the T cell receptor (TCR), CD8+ T cells undergo differentiation into distinct effector or memory T cell subsets, characterized by their differential expression of phenotypic markers, including CD127 (the IL-7 receptor) and KLRG1 (lectin-like receptor G1). Enhanced CD127 levels are indicative of effector CD8 T cells that are poised to differentiate into memory T cells. In contrast, effector cytotoxic CD8+ cells exhibit a phenotype characterized by altered levels of KLRG-1high/CD127low [17,18]. Given that HIF1α and associated metabolic pathways have previously been implicated in the regulation of effector and memory T cell differentiation [14,19], the levels of CD127+ KLRG1+ cells were assessed to gain insights into these processes. Remarkably, upon exposure to PMA and ionomycin, a notably increased population of cells with a KLRG-1high phenotype was observed within the CD8+ CD127+ cell subset from T-cell-specific HIF1α knockout mice [1]. KLRG1 is a cellular marker for short-lived effector CD8+ T cells (SLEC). However, it was designated that Tmem populations can be derived from either SLECs or Memory Precursor Effector Cells (MPECs). The downregulation of KLRG1 is not necessary for the formation of memory cells [20]. Furthermore, Long-Lived Effector Cells (LLECs) constitute a unique population that combines characteristics of both the effector and memory phases of the immune response and primarily originate from a subset of fate-permissive effector T cells [21]. In our study, it was observed that CD8+ T cells from T-HIF1α-/- mice exhibited heightened levels of IFN-γ and Tbx21 (T-bet) mRNA, demonstrating their enhanced potential to contribute to the anti-tumor response actively [1]. However, the reasons for these differing conclusions between our study and others remain unclear. This discordance highlights the need for a more comprehensive investigation into HIF1α expression and activity throughout T cells' dynamic metabolic life cycle within diverse tissues. Further research is needed to reconcile these conflicting findings and better understand the role of HIF1α in T-cell biology.
Under hypoxic tumor microenvironment conditions, T cells employ hypoxia-sensitive elements, such as HIF1α, to orchestrate transcriptional and post-translational programs in T cells. One notable gene regulated in response to hypoxia is CD39 (Entpd1), a pivotal member of the adenosine pathway and an ectoenzyme that consistently serves as a reliable marker for identifying tumor antigen-experienced T-exhausted cells (TEX) in both murine and human diseases [22]. Recent evidence indicates that hypoxia plays a significant role in TEX cells by elevating the expression of CD39 in CD8+ TEX cells in an HIF1α-dependent manner [5,22,23]. It was revealed that treating mice with axitinib and metformin, two agents targeting tumor hypoxia, provides therapeutic benefit by significantly reducing tumor hypoxia in mice and reducing suppressive activity CD8+ TEX, supporting the role of tumor hypoxia in TEX cell suppressor function [23]. Surprisingly, Foxp3+ Treg cells from the same tumor microenvironments (axitinib- and metformin-treated tumors) maintain their potent inhibitory properties ex vivo despite targeting hypoxia in the tumor microenvironment [23]. Similar to these findings, our study observed that despite the improved anti-tumor immune response with elevated effector function of CD8+ T cells after the inhibition of HIF1α in tumor-bearing mice, there was still an increased population of Foxp3+ Tregs [1].
It was summarized that Foxp3 expressing Tregs play a dual role in tumor immunity, with their immunosuppressive functions dampening anti-tumor responses and contributing to immune evasion by cancer cells. They maintain immune tolerance, which can extend to tumor cells, facilitating tumor progression [24]. However, efforts to selectively target or modulate Tregs in the tumor microenvironment are being explored to unleash anti-tumor immune responses. Combination therapy involving the modulation or depletion of Tregs in cancer treatment presents a multifaceted advantage by unleashing potent anti-tumor immunity. This approach synergizes with existing immunotherapies, overcomes resistance, minimizes systemic side effects, and offers personalized treatment tailored to individual immune profiles, and tumor characteristics [25,26]. In order to address the challenge posed by the elevated Treg population hindering the attainment of a potent anti-tumor immune response, a combination therapy approach was employed. This involved using both the HIF1α inhibitor, ACF, and Treg depletion reagent, such as Cytoxan [27,28]. This combinational therapeutic approach aimed to target the Tregs and the HIF1α underlying mechanisms, potentially enhancing the effectiveness of anti-tumor immune responses. Intriguingly, consistent with our previous findings, mice treated with ACF exhibited significantly decelerated tumor growth rates compared to the control group receiving the vehicle treatment. Similarly, the use of Cytoxan as a monotherapy also led to a notable reduction in tumor growth. Importantly, in alignment with our initial hypothesis, the combination of the HIF1α inhibitor with periodic low-dose Cytoxan yielded an even more substantial suppression of tumor development. In fact, the tumors in these mice were barely detectable for a significant portion of the experiment's duration [1]. These collective findings underscore the significant roles played by HIF1α in distinct subsets of CD8+ T cells. Importantly, our research suggests substantial implications for augmenting the potential of T cell-based anti-tumor immunity through the strategic combination of HIF1α and Treg inhibitors.
In conclusion, our recent study underscores the significant role played by HIF1α in the function of CD8+ T cells. It has been validated that T cell-specific deletion of HIF1α significantly reduces tumor development, accompanied by enhanced memory and inflammatory potential within CD8+ T cells. Moreover, our study suggests that combinational therapy employing both Treg and HIF1α inhibitors can effectively delay tumor development. These findings emphasize the detrimental effect of HIF1α on productive T cell responses to tumors and highlight the potential therapeutic value of HIF1α-blocking agents and Treg inhibitors in the treatment of aggressive cancers. Additionally, the study highlights the distinct effects of HIF1α within different T-cell subsets, offering opportunities for targeted interventions with substantial anti-tumor effects.
Acknowledgements
This work was supported by the National Key R&D Program of China (2022YFC2403000) and (2021YFC2400500), the National Natural Science Foundation of China (Grant 32170925), Shenzhen Science and Technology Program (KQTD20210811090115019), the Shenzhen Science and Technology Program (JCYJ2022081800807016), the startup fund of SIAT and CAS. Ping Wei’s work was supported by the Natural Science Foundation of Chongqing Grant CSTB2022NSCQ-MSX1069, the Chongqing for overseas Scholars Grant CX2022118.
Author Contributions
FR and PW drafted the manuscript; FP outlined and critically revised the manuscript.
Conflict of Interests
The authors declare no competing interests.
References
2. Turner SJ, Bennett TJ, La Gruta NL. CD8(+) T-Cell Memory: The Why, the When, and the How. Cold Spring Harbor Perspectives in Biology. 2021;13(5):a038661.
3. Philip M, Schietinger A. CD8(+) T cell differentiation and dysfunction in cancer. Nature Reviews Immunology. 2022;22(4):209-23.
4. Kao KC, Vilbois S, Tsai CH, Ho PC. Metabolic communication in the tumour-immune microenvironment. Nature Cell Biology. 2022;24(11):1574-83.
5. Scharping NE, Rivadeneira DB, Menk AV, Vignali PDA, Ford BR, Rittenhouse NL, et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nature Immunology. 2021;22(2):205-15.
6. Luo Z, Tian M, Yang G, Tan Q, Chen Y, Li G, et al. Hypoxia signaling in human health and diseases: implications and prospects for therapeutics. Signal Transduction and Targeted Therapy. 2022;7(1):218.
7. Todorović L, Stanojević B. VHL tumor suppressor as a novel potential candidate biomarker in papillary thyroid carcinoma. Biomolecules & Biomedicine. 2023;23(1):26-36.
8. Li Y, Patel SP, Roszik J, Qin Y. Hypoxia-Driven Immunosuppressive Metabolites in the Tumor Microenvironment: New Approaches for Combinational Immunotherapy. Front Immunol. 2018;9:1591.
9. Corbet C, Feron O. Tumour acidosis: from the passenger to the driver's seat. Nature Reviews Cancer. 2017;17(10):577-93.
10. Singleton DC, Macann A, Wilson WR. Therapeutic targeting of the hypoxic tumour microenvironment. Nature Reviews Clinical oncology. 2021;18(12):751-72.
11. Gropper Y, Feferman T, Shalit T, Salame TM, Porat Z, Shakhar G. Culturing CTLs under Hypoxic Conditions Enhances Their Cytolysis and Improves Their Anti-tumor Function. Cell Reports. 2017;20(11):2547-55.
12. Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, et al. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nature Immunology. 2013;14(11):1173-82.
13. Palazon A, Tyrakis PA, Macias D, Veliça P, Rundqvist H, Fitzpatrick S, et al. An HIF-1α/VEGF-A Axis in Cytotoxic T Cells Regulates Tumor Progression. Cancer Cell. 2017;32(5):669-83.e5.
14. Liikanen I, Lauhan C, Quon S, Omilusik K, Phan AT, Bartrolí LB, et al. Hypoxia-inducible factor activity promotes antitumor effector function and tissue residency by CD8+ T cells. The Journal of Clinical Investigation. 2021;131(7):e143729.
15. Parczyk J, Ruhnau J, Pelz C, Schilling M, Wu H, Piaskowski NN, et al. Dichloroacetate and PX-478 exhibit strong synergistic effects in a various number of cancer cell lines. BMC Cancer. 2021;21(1):481.
16. Luo F, Lu FT, Cao JX, Ma WJ, Xia ZF, Zhan JH, et al. HIF-1α inhibition promotes the efficacy of immune checkpoint blockade in the treatment of non-small cell lung cancer. Cancer Letters. 2022;531:39-56.
17. Remmerswaal EBM, Hombrink P, Nota B, Pircher H, Ten Berge IJM, van Lier RAW, et al. Expression of IL-7Rα and KLRG1 defines functionally distinct CD8(+) T-cell populations in humans. European Journal of Immunology. 2019;49(5):694-708.
18. Herndler-Brandstetter D, Ishigame H, Shinnakasu R, Plajer V, Stecher C, Zhao J, et al. KLRG1(+) Effector CD8(+) T Cells Lose KLRG1, Differentiate into All Memory T Cell Lineages, and Convey Enhanced Protective Immunity. Immunity. 2018;48(4):716-29.e8.
19. Liu R, Muliadi V, Mou W, Li H, Yuan J, Holmberg J, et al. HIF-1 stabilization in T cells hampers the control of Mycobacterium tuberculosis infection. Nature Communications. 2022;13(1):5093.
20. Renkema KR, Huggins MA, Borges da Silva H, Knutson TP, Henzler CM, Hamilton SE. KLRG1(+) Memory CD8 T Cells Combine Properties of Short-Lived Effectors and Long-Lived Memory. Journal of Immunology (Baltimore, Md : 1950). 2020;205(4):1059-69.
21. Youngblood B, Hale JS, Kissick HT, Ahn E, Xu X, Wieland A, et al. Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature. 2017;552(7685):404-9.
22. Canale FP, Ramello MC, Núñez N, Araujo Furlan CL, Bossio SN, Gorosito Serrán M, et al. CD39 Expression Defines Cell Exhaustion in Tumor-Infiltrating CD8(+) T Cells. Cancer Research. 2018;78(1):115-28.
23. Vignali PDA, DePeaux K, Watson MJ, Ye C, Ford BR, Lontos K, et al. Hypoxia drives CD39-dependent suppressor function in exhausted T cells to limit antitumor immunity. Nature Immunology. 2023;24(2):267-79.
24. Lu L, Barbi J, Pan F. The regulation of immune tolerance by FOXP3. Nature Reviews Immunology. 2017;17(11):703-17.
25. Raffin C, Vo LT, Bluestone JA. Treg cell-based therapies: challenges and perspectives. Nature Reviews Immunology. 2020;20(3):158-72.
26. Dwivedi M, Tiwari S, Kemp EH, Begum R. Implications of regulatory T cells in anti-cancer immunity: from pathogenesis to therapeutics. Heliyon. 2022;8(8):e10450.
27. Scurr M, Pembroke T, Bloom A, Roberts D, Thomson A, Smart K, et al. Low-Dose Cyclophosphamide Induces Antitumor T-Cell Responses, which Associate with Survival in Metastatic Colorectal Cancer. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research. 2017;23(22):6771-80.
28. Yan W, Lang T, Zhu R, Zhu X, Li Y, Wu T, et al. Anti-hypoxia nanosized drug delivery systems improving cancer therapy. Nano Today. 2022;42:101376.