Abstract
Multicentric reticulohistiocytosis (MRH) is a rare disease affecting skin and joints and, resulting in erosive arthritis. The association of MRH and other autoimmune diseases is not negligible. We present a review of the literature of the cases associating both conditions between 1985 and 2021. All the selected cases of MRH had to be confirmed histologically and associated to other autoimmune diseases. This article is aimed at physicians who care for patients with polyarthritis, to highlight the distinctive features that differentiate MRH from the common arthritides.
Keywords
Multicentric reticulohistiocytosis, Autoimmune diseases, Rheumatology, Arthritis, Skin disease
Introduction
Multicentric reticulohistiocytosis (MRH) is a rare granulomatous disease manifesting by the subsequent appearance of erosive aggressive polyarthritis and typical skin papulonodular lesions [1]. It can also affect internal organs such as the lungs, hearts in addition to rare cases of mesenteric lymphadenopathy and urogenital lesions. There are approximately 300 cases of reported MRH [2]. The vast majority of them come from Caucasian ethnicity [2]. The mean age of presentation is usually seen in the fourth, although it has been reported both in children and elderly people [3]. Although no definite etiology has been established, the underlying pathology is assumed to originate from the phagocytosis of histiocytes [4]. There are no definitive diagnostic laboratory tests for MRH, as the definitive diagnosis is based on histologic findings [5]. MRH can also be associated with other systemic autoimmune diseases, making the diagnosis challenging. In this article, we present a comprehensive review of the literature of MRH cases associated with other autoimmune diseases.
Methods
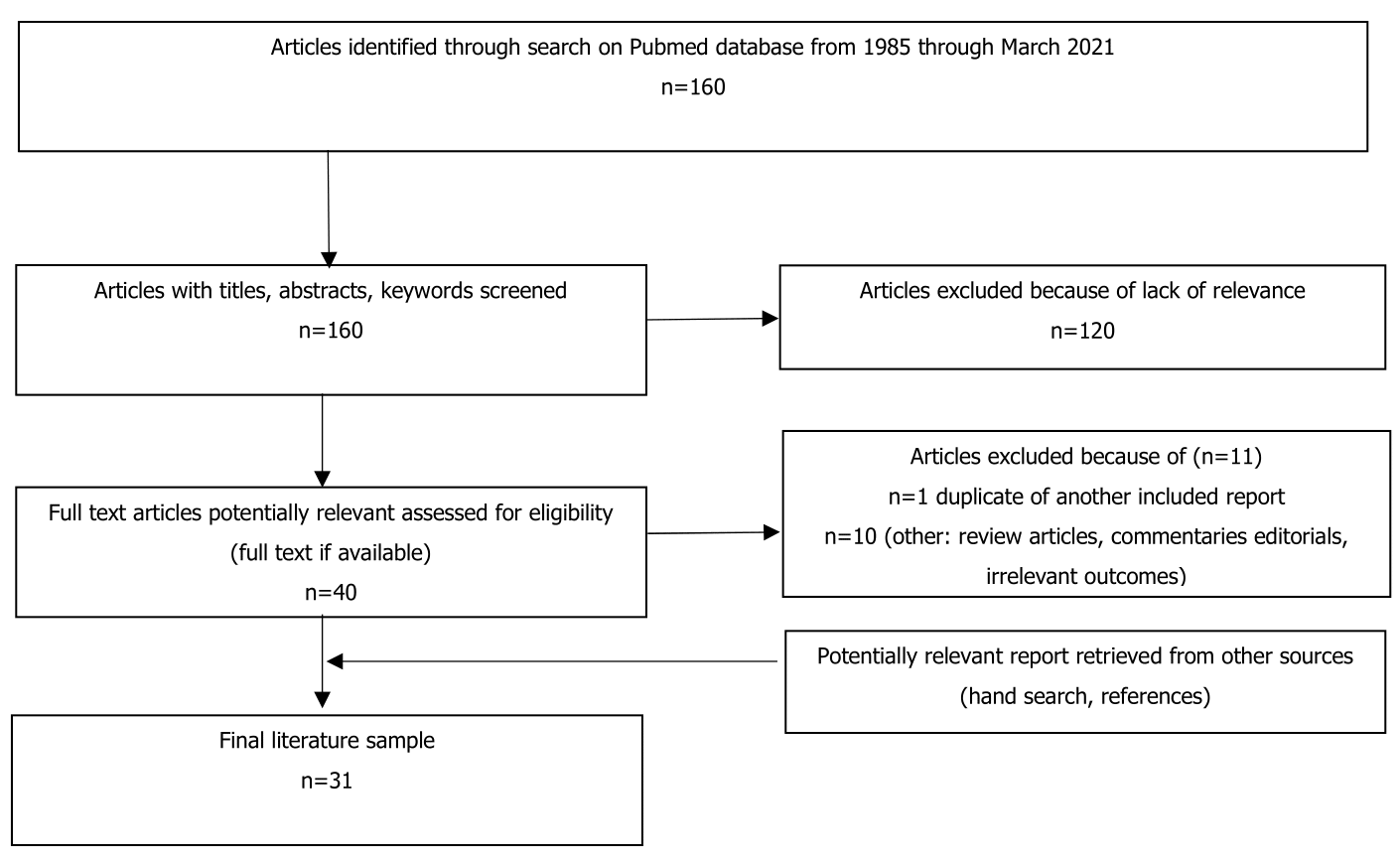
We conducted a review of the literature of cases associating MRH with autoimmune diseases. PubMed was searched from 1980 through March 2021 using the search term ‘multicentric reticulohistiocytosis’ and (rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, systemic sclerosis and dermatomyositis) yielding 160 articles. Articles in which the diagnosis of MRH was clear, but the association of other autoimmune disease was not mentioned were excluded. We screened for inclusion criteria including a co-occurrence of MRH with autoimmune diseases of articles written in the English language (Figure 1) and in which the diagnosis of the autoimmune disease was based on accepted criteria: the American College of Rheumatology or/and the European League Against Rheumatism. Moreover, as the diagnoses of MRH required a biopsy, all the selected cases must be confirmed histologically.
Figure 1. Flowchart outlining the protocol adopted in this review.
Results
A total of 31 articles were included (case reports n=30, retrospective study (n=1)) (Table 1). For the purpose of this review, cases will be divided according to the corresponding autoimmune disease.
|
The autoimmune disease |
Number of cases |
|
Rheumatoid arthritis [1-17] |
17 |
|
Sjögren’s syndrome [18-20] |
6 |
|
Systemic lupus erythematosus [21-23] |
2 |
|
Systemic sclerosis [24] |
1 |
|
Dermatomyositis [25] |
3 |
|
Undifferentiated connective tissue disease [34] |
1 |
Rheumatoid arthritis
In the literature, three cases reported a co-occurrence of rheumatoid arthritis (RA) and MRH. The first case described a patient with polyarthritis sparing the distal interphalangeal (DIP) joints and skin lesions. The rheumatoid factor (RF), and anti-cyclic citrullinated peptide antibody (anti-CCP) were positive. The histologic findings of the papulonodular biopsy were consisted with MRH [6]. The second case was reported in a patient with a history of MRH treated with cyclophosphamide. Three years after stopping treatment, the patient developed joint pain consistent with RA and was found to be anti-cyclic citrullinated peptide (anti-CCP) positive [7]. Ben Abdelghani et al., also reported an overlap case of MRH and RA complicated with Sjögren’s syndrome [8]. Three other cases with high anti-CCP were reported in the literature [3,9,10]. In the case of Chahan et al., the patient was initially diagnosed with RA. She presented with symmetric DIP joint destruction and papules along her nail beds [9]. Histologic findings confirmed the diagnosis of MRH [9]. The possibilities of MRH with positive rheumatoid serology or coexistence of multiple cutaneous reticulohistiocytomas and RA are discussed [11]. On the contrary, some cases of MRH have been reportedly misdiagnosed as RA [12-17]. Horvath and Hoffman reported a case of MRH, which was subsequently found to be misdiagnosed as RA and initially treated with naproxen and hydroxychloroquine [14]. In another case, MRH was misdiagnosed as RA but based on the DIP involvement and skin findings, the patient was diagnosed with MRH [14]. Saba et al., also reported a case of a 54-year-old female who was misdiagnosed as having RA and underwent numerous joint replacement surgeries. The patient finally received a diagnosis of MRH after the histopathological examination of the patient’s left knee arthroplasty which revealed a diffuse histiocytic infiltrate, multinucleated giant cells, and finely granulated eosinophilic cytoplasm with a ground-glass appearance [17].
Sjögren’s syndrome
The existing literature contains six cases of co-occurrence of MRH and Sjögren’s syndrome (SS). In most of these cases, skin changes are accompanied by arthritis, typically of a destructive nature. In one case, the patient had xerostomia and xerophtalmia. The antinuclear antibody (ANA) titer was 1/640 in a discrete speckled pattern, with high positive extractable nuclear antibodies to Ro and bilaterally positive Schirmer’s test [18]. In another case, the diagnosis of SS was made after 18 years of MRH diagnosis; when the patient developed chronic destructive symmetrical arthritis and xerostomia. Biopsies of the synovium and minor salivary glands established a diagnosis of MRH polyarthritis and lymphocytic infiltrative SS, respectively. Anti-Ro antibody was also present [19]. The case by Shiokawa et al. also reported a co-occurrence of both condition [20]. The patient presented with recurrent left parotid gland enlargement, positive anti-Ro antibodies, positive Schirmer’s test and positive sialography findings [20]. In another study, the diagnosis of SS and MRH was made in a patient with polyarthralgia, papulonodular lesions of the dorsum of the hands, periungual regions, and xerostomia. Serologic findings included a positive ANA titer 1/480, with a speckled immunofluorescent pattern and a positive SS-A (Ro). The biopsy of a nodule on the dorsum of the patient hand revealed dense proliferation of fibrohistiocytic and multinucleated giant cells with ground-glass cytoplasm. The biopsy of the salivary glands showed periacinal and periductal plasmacellular and lymphocytic exudate, typical for Sjögren’s syndrome [21]. Finally, a 50-year-old woman who had been diagnosed as suffering from RA with positive RF and anti-CCP, presented 4 years after the diagnosis, multiple asymptomatic papulonodular eruption. The histopathologic examination was suggestive of MRH. She developed concomitantly SS with systemic manifestations [8].
Lupus
Only two cases were reported in the literature. In one case, the diagnosis of systemic lupus erythematosus (SLE) complicated with MRH was certain. The patient developed a brown tumor over the right ankle area. The latter was biopsied and diagnosed as MRH based on histological features, including accumulation of histiocytes and multinucleated cells in the tissue. Treatment with cyclosporine A induced a marked regression of these nodules within one month, with a complete remission of both MRH and lupus nephritis [22]. In another case, the diagnosis of lupus was less certain. The patient, who had polyarthritis, developed fixed erythematous plaques consistent with subacute cutaneous lupus erythematosus. The ANA titer was 1/1280, with homogenous pattern. The further serologic profile was negative. A biopsy of the skin confirmed the diagnosis of MRH after 2 years of the arthritis onset [23].
Systemic sclerosis
Only one case associating systemic sclerosis (SSc) and MRH was described in the literature. Takashi et al. reported a case of a 42-year-old Japanese woman who complained of tender nodules on the left hand, fingers with skin sclerosis polyarthralgia and xerostomia [24]. The serum antinuclear antibody test revealed anti-centromere antibody and a discrete speckled pattern of anti-nuclear antibody. The biopsy from the finger nodule and the sclerotic finger skin showed a perivascular infiltration of multinucleated giant cells with ground-glass cytoplasm and dermal thick collagen proliferation, respectively. The lip biopsy and sialography specimens showed periductal lymphocyte infiltration and apple tree-like changes. Systemic corticosteroid treatment improved the polyarthritis, xerostomia, and skin sclerosis rapidly but suppressed the nodular lesions only gradually [24].
Dermatomyositis
MRH was determined in the course of systemic dermatomyositis (DM) in 3 cases [25]. An association of polymyositis with MRH was also found in a male patient with skin nodules [26]. The cutaneous findings included an erythematous, predominantly photodistributed macular and papular eruption clinically consistent with DM [27-29]. In other cases, the presence of the photo-distributed eruption and the presence of papulonodular lesions on the dorsum of the hands and interphalangeal joints, mimicking Gottron papules with periungual telangiectasia, caused the confusion of MRH with DM [25, 30-32]. While most of the reported cases of MRH confused with DM were based on dermatological features of amyopathic DM, two cases were associated with muscle weakness [26,29].
The findings of the histopathological examination revealed in all cases dermal infiltrates of multinucleated histiocytic giant cells with an eosinophilic ‘ground glass’ cytoplasm consisting with the diagnosis of MRH. This suggests that these patients are not presenting with a DM-MRH overlap syndrome, but rather MRH with clinical findings reminiscent of dermatomyositis.
Both diagnoses have been associated with malignancy; therefore, patients with either disease process should have age-appropriate and symptom-directed screening for malignancy [27].
Other autoimmune diseases
One case exhibited a positive RF, anti-SSA/Ro, and anti-SSB/La. The patient had peripheral and sacroiliac joint involvement as well as skin manifestations. The diagnosis of MRH was suggested by skin biopsies. Sacroiliac joint involvement has previously been reported in MRH [33]. Recently, Niklas et al. also reported a case of undifferentiated connective tissue disease (UCTD) associated with MRH without destructive lesions in the joints [34]. The immunologic profile showed a positive RF, anti-CCP, and antinuclear antibodies of titer 1/ 1280 with the presence of the SSA and SSB antibodies. The patient was started on chloroquine at a dosage of 250 mg/day [34]. In a study by Alvarez et al. including 24 patients with biopsy-proven MRH, 39% of patients had a concomitant autoimmune disease. Furthermore, a positive autoimmune serology was found in 44% for ANA, 28% for RF, 44% for Anti-CCP and 75% for anti-Ro antibody [41].
Discussion
MRH manifests with two major clinical features. It includes an aggressive erosive peripheral polyarthritis generally preceding the skin phenotype by an average of 3 years [4]. Arthritis affects hands and wrists usually bilaterally and symmetrically, but also the large joints and sometimes cervical spine. The most common involvement of the interphalangeal joint of the hand is the distal interphalangeal joint (DIP), accounting for 75% of patients [3]. As the disease progresses, joint space narrowing of the distal DIP, marginal erosions, and absence of new bone formation is a unique feature of MRH. Joint space narrowing and periarticular osteopenia may also be present [3].
Symptoms of arthritis may rapidly progress to severe joint destruction, leading in 45% of cases to arthritis mutilants and opera-glass hand [6]. The articular features can also be self-limiting or relapsing-remitting over time in less aggressive cases [4]. Cutaneous involvement occurs initially in 18% of patients, and more commonly follows the involvement of the joint [5]. The skin lesions consist of multiple, various-sized, asymptomatic, and erythematous to brown papulonodules mainly locating in the upper half of the body, especially the face and hands with a predilection for the periungeal area, mimicking “coral bed” appearance [8]. In other cases, Patients may present with photodistributed macular eruptions on the face, the V of the neck, or the forearms. Atypical skin lesions include macular photodistributed erythema, periungual telangiectasia, and xanthelasma-like lesions [35]. Patients may complain of pruritus but pain is generally absent [4]. Constitutional symptoms including fever, fatigue, and weight loss are frequently seen in MRH [35]. Other organs may be affected in particular, heart and lung manifestations may occur with pleural and pericardial effusions, leading in some cases to heart failure or pulmonary fibrosis [35]. Mucosal involvement is observed in the head and neck district, but also of the urinary tract [35]. Furthermore, the involvement of the liver, abdominal or urogenital lymph nodes, muscles, bones, thyroid glands has been occasionally reported [4]. Two case reports of MRH with thrombosis have been reported [35,36].
Differential diagnoses and associated diseases
MRH has been reported to be associated with several malignant conditions in 25% of cases [4]. MRH may be associated with hematologic malignancies such as lymphoma, leukemia and myelodysplastic syndrome as well as solid and cutaneous neoplasm (lung, laryngeal, bronchial, ovarian, endometrial, cervix, stomach, colon, liver, mesothelioma, sarcoma, malignant melanoma, renal, bladder, penile, thyroid, and breast cancers) [8]. However, it is still debated whether MRH should be regarded as a paraneoplastic manifestation. While some cases reported the improvement of MRH skin and joint manifestations after cancer remission [37, 38], other cases were associated with a metastatic occult cancer [39].
Additional non autoimmune diseases have been occasionally associated with MRH and include diabetes mellitus, thyroid disease, colitis, and dyslipidemias [2]. Finally, TB infection has been associated with MRH occurrence, and the use of antitubercular agents has been suggested in some cases [40].
Autoimmune diseases
Distinguishing between RA and MRH may be difficult especially when only arthritis is present without skin manifestations. From a clinical standpoint, the DIP joint and skin involvement favors the diagnosis of MRH, whereas RA preferentially involves the proximal joints of the hands [15]. Joints with RA involvement tend to have a more insidious pattern of joint destruction and more prominent destruction of cartilage and juxtaarticular osteoporosis, whereas MRH-affected joints tend to have wide articular cavities as a result of subarticular bone reabsorption [15]. In MRH, RF and anti-CCP tests are usually negative. The low incidence of this association explains the lack of recommendation. Treatment continues to be experimental and includes methotrexate, non-steroidal anti-inflammatory drugs, hydroxychloroquine, corticosteroid and leflunomide [6].
Seronegative arthritis, including psoriatic arthritis, may also be a differential diagnosis but the lack of axial involvement and skin psoriasis in MRH contribute to discriminate between the two conditions [2]. Also, the presence of psoriatic plaques, nail changes, entheseal and periosteal new bone production, ankylosis and an asymmetrical pattern of joint involvement is in favor of psoriatic arthritis [3].
Gouty arthritis may also involve the DIP joints. However, unlike MRH, gout is usually associated with soft-tissue swelling and central, marginal, or periarticular erosions with overhanging edges and reactive changes in the bone [35]. Moreover, skin involvement is not a typical finding of gout and when present it is generally limited to the skin around the involved joints. The diagnosis of MRH is ruled out by synovial fluid examination and the identification of typical urate crystals by polarized light microscopy [35].
In erosive osteoarthritis, erosion is commonly sharply marginated in the central portion of the joint with the presence of marginal osteophytes and intra-articular bony ankylosis, the 'sea gull wing' pattern [35].
In addition to RA, MRH is also associated in 5-20% of cases with other systemic autoimmune diseases including SLE, SS and SSc [3]. Many reported cases were also misdiagnosed as DM du to the cutaneous manifestations. However, the typical rashes are the heliotrope rash, malar erythema and poikiloderma and they are not mainly composed of papules [32]. Moreover, the distribution of papules on the dorsum of the hands is different. Gottron’s papules are found over the knuckles [33]. However, the papules or nodules of the hands in MRH occur on the dorsum and lateral aspects of fingers and nail folds [33]. Finally, the musculoskeletal symptoms in MRH are different as opposed to those of proximal muscle weakness seen in DM. On this occasion, the absence of muscle weakness, evaluation of muscle enzymes and electromyography can help differentiate MRH from DM [26,29]. The histologic finding of osteoclast-like multinucleated giant cells in MRH is easily differentiated from the interface dermatitis and superficial and deep perivascular infiltrate seen in dermatomyositis. This straightforward distinction supports the need for biopsy prior to making a diagnosis of dermatomyositis.
The association between MRH and autoimmune diseases may suggest a pathogenic relationship which is difficult to ascertain, given the lack of data concerning MRH pathogenesis [8]. However, it could be assumed that cellular and/or humoral autoimmune mechanisms could induce the activity of histiocytic cells [2]. Indeed, overexpression of the proinflammatory cytokines (IL-1, IL-6, IL-12, and TNF-a) might possibly be involved in the pathogenesis of MRH. Thus, MRH could be induced by an autoimmune disease, a neoplasm, an infection or other unknown factors [8].This severe disease warrants immunomodulatory treatment.
Treatment modalities
Given the rarity and infrequent presentation of this disease, as well as the lack of knowledge of its pathogenesis, there are no established treatment guidelines for the management of MRH. Thus, the lack of sufficient patient cohort for clinical trials, allows only to evaluate treatment efficacy in limited case reports.
A combination of corticosteroids and methotrexate is recommended in mild to severe disease and seems effective in controlling arthritis [7]. Some therapeutic success has been achieved with other immunosuppressive agents and immunomodulators such as azathioprine hydroxychloroquine, leflunomide, and cyclophosphamide especially in severe and refractory cases [41].
Bisphosphonates including alendronate, zoledronic acid, and pamidronate have also been used as monotherapy or as combination therapy with conventional disease-modifying antirheumatic drugs (DMARDs) [35].
Experience with bisphosphonates has generally been effective on both skin and joint involvement suggesting that MRH may be a systemic osteoclastic disease [6]. A proposed mechanism of action involves the receptor activator of nuclear factor kappa B ligand (RANKL) that has been shown to be expressed on histiocytes in the skin and joints in MRH [35]. In this context, a good response to Denosumab, a monoclonal antibody against RANK-L, was obtained in a patient with MRH [35]. However, further trials are needed to confirm Denosumab efficiency.
Recently, biological treatments have been used as a second-line option including infliximab, etanercept, adalimumab, golimumab, tocilizumab, and anakinra have been used in patients with MRH with a beneficial effect on skin and articular manifestation and systemic with IL1 [35,41].
Whether the disease has a neoplastic basis and could benefit from targeted chemotherapy remains unclear and warrants further study [41].
Prognosis
MRH may typically surrender spontaneously after several years of evolution with or without treatment, within an average of 8 years [35]. The prognosis of MRH is directly related to the underlying malignancy when there is an associated tumor. Destructive arthritis can also lead to functional impairment [3].
To our knowledge, this is the first review focusing on the association of MRH with other autoimmune diseases. Another strength is that we reviewed clinical features and treatment options. The comparison between the different treatments was difficult as no comparative randomized controlled studies were performed. Despite these clear limitations, the publication of more cases associating both conditions would improve our knowledge for an earlier recognition. Further studies are needed in order to establish clear guidelines for an optimal choice of treatment.
Conclusion
MRH is an exceedingly rare systemic illness. Even though this condition has some unique clinical and radiographic features, diagnosis can be challenging, especially when associated with other autoimmune inflammatory conditions. Indeed, this association is real and more common than just coincidence. The most frequently associated autoimmune conditions were RA and GJS. A definitive diagnosis of MRH rests upon histological examination. Owing to its rarity, there are no established treatment guidelines for the management of MRH. Prompt recognition and early treatment are essential because if left untreated, MRH can lead to progressive joint destruction and disability.
Declaration
All the authors contributed in drafting and writing the article.
Conflicts of Interest
None.
References
2. Selmi C, Greenspan A, Huntley A, Gershwin ME. Multicentric reticulohistiocytosis: a critical review. Curr Rheumatol Rep. 2015;17(6):511.
3. Tariq S, Hugenberg ST, Hirano-Ali SA, Tariq H. Multicentric reticulohistiocytosis (MRH): case report with review of literature between 1991 and 2014 with in depth analysis of various treatment regimens and outcomes. Springerplus. 2016;25;5:180.
4. Gorman JD, Danning C, Schumacher HR, Klippel JH, Davis JC Jr. Multicentric reticulohistiocytosis: case report with immunohistochemical analysis and literature review. Arthritis Rheum. 2000;43(4):930-8.
5. Macía-Villa CC, Zea-Mendoza A. Multicentric reticulohistiocytosis: case report with response to infliximab and review of treatment options. Clin Rheumatol. 2016;35(2):527-34.
6. Behera A, Devi S, Guru S, Sethy M. A Rare Case of Multicentric Reticulohistiocytosis with Concurrent Rheumatoid Arthritis. Cureus. 2019;24;11(8):e5476.
7. Kumar B, Singh N, Rahnama-Moghadam S, Wanat KA, Ijdo JW, Werth VP. Multicentric Reticulohistiocytosis: A Multicenter Case Series and Review of Literature. J Clin Rheumatol. 2018 ;24(1):45-9.
8. Ben Abdelghani K, Mahmoud I, Chatelus E, Sordet C, Gottenberg JE, Sibilia J. Multicentric reticulohistiocytosis: an autoimmune systemic disease? Case report of an association with erosive rheumatoid arthritis and systemic Sjogren syndrome. Joint Bone Spine. 2010;77(3):274-6.
9. Chauhan A, Mikulik Z, Hackshaw KV. Multicentric reticulohistiocytosis with positive anticyclic citrullinated antibodies. J Natl Med Assoc. 2007;99(6):678-80.
10. Codriansky KA, Rünger TM, Bhawan J, Kantarci A, Kissin EY. Multicentric reticulohistiocytosis: a systemic osteoclastic disease? Arthritis Rheum. 2008;15;59(3):444.
11. Vieira R, Cordeiro MR, Mariano A, Reis JP, Tellechea O, Figueiredo A. Multiple cutaneous reticulohistiocytomas in a patient with rheumatoid arthritis. Dermatol Online J. 2004;15;10(2):11.
12. Hoshina D, Shimizu T, Abe R, Murata J, Tanaka K, Shimizu H. Multicentric reticulohistiocytosis associated with rheumatoid arthritis. Rheumatol Int. 2005;25(7):553-4.
13. Krause ML, Lehman JS, Warrington KJ. Multicentric reticulohistiocytosis can mimic rheumatoid arthritis. J Rheumatol. 2014;41(4):780-1.
14. Horvath JR, Hoffman GS. Multicentric reticulohistiocytosis: a mimic of gout and rheumatoid arthritis. Cleve Clin J Med. 1999;66(3):166-72.
15. Keum DI, Yoon NY, Lee WS. Multicentric reticulohistiocytosis misdiagnosed as rheumatoid arthritis. Int J Dermatol. 2015;54(6):e222-5.
16. Kumar A, Bhat A, Misra R, Malaviya AN, Suri RK, Sohi AS. Multicentric reticulohistiocytosis mimicking rheumatoid arthritis. Clin Exp Rheumatol. 1985;3(4):360-1.
17. Saba R, Kwatra SG, Upadhyay B, Mirrakhimov AE, Khan FN. Multicentric reticulohistiocytosis presenting with papulonodular skin lesions and arthritis mutilans. Case Rep Rheumatol. 2013;2013:201563.
18. Morris-Jones R, Walker M, Hardman C. Multicentric reticulohistiocytosis associated with Sjögren's syndrome. Br J Dermatol. 2000;143(3):649-50.
19. Carey RN, Blotzer JW, Wolfe ID, Glusman SM, Arnett FC. Multicentric reticulohistiocytosis and Sjögren's syndrome. J Rheumatol. 1985;12(6):1193-5.
20. Shiokawa S, Shingu M, Nishimura M, Yasuda M, Yamamoto M, Tawara T, et al. Multicentric reticulohistiocytosis associated with subclinical Sjögren's syndrome. Clin Rheumatol. 1991 ;10(2):201-5.
21. René J, Starz T, Miller EB, Winkelstein A. Multicentric reticulohistiocytosis and Sjögren's syndrome. Arthritis Rheum. 1990;33(12):1870-1.
22. Saito K, Fujii K, Awazu Y, Nakayamada S, Fujii Y, Ota T, Tanaka Y. A case of systemic lupus erythematosus complicated with multicentric reticulohistiocytosis (MRH): successful treatment of MRH and lupus nephritis with cyclosporin A. Lupus. 2001;10(2):129-32.
23. Badlissi F, Setty Y, Folzenlogen D. A case of multicentric reticulohistiocytosis initially misdiagnosed as lupus. J Clin Rheumatol. 2002;8(4):232-3.
24. Takahashi M, Mizutani H, Nakamura Y, Shimizu M. A case of multicentric reticulohistiocytosis, systemic sclerosis and Sjögren syndrome. J Dermatol. 1997;24(8):530-4.
25. Hsiung SH, Chan EF, Elenitsas R, Kolasinski SL, Schumacher HR, Werth VP. Multicentric reticulohistiocytosis presenting with clinical features of dermatomyositis. J Am Acad Dermatol. 2003 ;48(2 Suppl):S11-4.
26. Anderson TE, Carr AJ, Chapman RS, Downie AW, Maclean GD. Myositis and myotonia in a case of multicentric reticulohistiocytosis. Br J Dermatol. 1968;80(1):39-45.
27. Fett N, Liu RH. Multicentric reticulohistiocytosis with dermatomyositis-like features: a more common disease presentation than previously thought. Dermatology. 2011;222(2):102-8
28. Mun JH, Ko HC, Kim MB. Multicentric reticulohistiocytosis masquerading as dermatomyositis: similar and different features. J Dermatol. 2012;39(1):104-7.
29. McIlwain KL, DiCarlo JB, Miller SB, Lim S. Multicentric reticulohistiocytosis with prominent cutaneous lesions and proximal muscle weakness masquerading as dermatomyositis. J Rheumatol. 2005 ;32(1):193-4.
30. Shima N, Murosaki T, Nagashima T, Iwamoto M, Amano Y, Nakano N, et al. Multicentric Reticulohistiocytosis with Dermatomyositis-like Eruptions. Intern Med. 2017;56(15):2063-6.
31. Muñoz-Santos C, Sàbat M, Sáez A, Gratacós J, Luelmo J. Multicentric reticulohistiocytosis-mimicking dermatomyositis. Case report and review of the literature. Dermatology. 2007;214(3):268-71.
32. Tait TJ, Bird HA, Ford GP. Multicentric reticulohistiocytosis: presentation with the cutaneous features of dermatomyositis. Br J Rheumatol. 1994;33(1):100-1.
33. Rezuș E, Burlui MA, Cardoneanu A, Haba D, Danciu M, Cozma RS, et al. Multicentric Reticulohistiocytosis Exhibiting Positive HLA-B*07 and HLA-B*08: A Case Report. Medicina (Kaunas). 2020;8;56(9):456.
34. Niklas K, Niklas A, Puszczewicz M. Multicentric reticulohistiocytosis in the course of undifferentiated connective tissue disease. Postepy Dermatol Alergol. 2019;36(5):646-7.
35. Toz B, Büyükbabani N, İnanç M. Multicentric reticulohistiocytosis: Rheumatology perspective. Best Pract Res Clin Rheumatol. 2016;30(2):250-60.
36. Rentsch JL, Martin EM, Harrison LC, Wicks IP. Prolonged response of multicentric reticulohistiocytosis to low dose methotrexate. J Rheumatol. 1998;25:1012e5.
37. Tan BH, Barry CI, Wick MR, White KP, Brown JG, Lee A, et al. Multicentric reticulohistiocytosis and urologic carcinomas: a possible paraneoplastic association. J Cutan Pathol. 2011;38:43-8.
38. Tirumalae R, Rout P, Jayaseelan E, Shet A, Devi S, Kumar KR. Paraneoplastic multicentric reticulohistiocytosis: a clinicopathologic challenge. Indian J Dermatol Venereol Leprology. 2011;77:318-20.
39. Worm M, Kleine-Tebbe A, von Stebut E, Haas N, Kolde G. Multicentric reticulohistiocytosis indicating metastasis of an unknown primary tumour. Acta Derm Venereol. 1998;78:67-8.
40. Gold KD, Sharp JT, Estrada RG, Duffy J, Person DA. Relationship between multicentric reticulohistiocytosis and tuberculosis. JAMA. 1977;237:2213-4.
41. Sanchez-Alvarez C, Sandhu AS, Crowson CS, Wetter DA, McKenzie GA, Lehman JS, et al. Multicentric reticulohistiocytosis: the Mayo Clinic experience (1980-2017). Rheumatology (Oxford). 2020;59(8):1898-905