Abstract
Pediatric convulsive status epilepticus is a neurological emergency requiring termination in a time critical fashion, if permanent brain damage or death are to be avoided. Levetiracetam has been suggested as a potential second line agent for benzodiazepine refractory seizures. This review will examine the evidence for levetiracetam’s efficacy and safety in the management of pediatric convulsive status epilepticus.
Keywords
Epilepsy, Childhood convulsive status epilepticus, Anti convulsants
Introduction
Convulsive status epilepticus (CSE) among children is a common serious neurological emergency associated with significant sequalae and mortality [1]. Benzodiazepines are used to terminate CSE in the first instance and are successful approximately 60% of the time [2-3]. There is no clear-cut consensus as to which agent to select when confronted with benzodiazepine refractory CSE. Traditionally phenytoin has been favored as the second line treatment for CSE but there is a paucity of evidence for its use. Phenytoin is also associated with some serious side effects and it is cumbersome to administer [2]. Levetiracetam has been suggested as a viable alternative to phenytoin as a second line agent in CSE [4]. A systematic review and meta-analyses were undertaken by the authors to examine the available evidence for the efficacy and safety of levetiracetam [5].
Methods
Several databases were searched including Medline and Cochrane Central Registrar of Controlled Trials. Randomized controlled trials that compared intravenous levetiracetam with other intravenous antiepileptic drugs used for CSE in children aged between 1 month and 18 years were identified and assessed. The primary outcome was the clinical cessation of CSE. Secondary outcome measures included; time to cessation of CSE, need for rapid sequence intubation, admission to intensive care, adverse events and all-cause mortality.
Data extraction was completed by the review authors working in pairs with disagreements adjudicated by an arbiter author once all eligible citations had been agreed upon. An assessment of bias was conducted on the extracted data based on the Cochrane Handbook for Systematic Reviews of Interventions.
For detailed information on data management, the reader is referred to the original article [5]. The findings were summarized using the Grading Recommendations Assessment Development and Evaluation system (GRADE) principles to assess the quality of the totality of the evidence associated with specific outcomes.
Results
Ten randomized control trials (RCTs) met the eligibility criteria out of over 20,000 citations reviewed. The RCTs were conducted across 4 continents and all the participants were aged between 1 month and 18 years. There were a total of 1907 children in the ten selected studies. Seven studies compared levetiracetam to phenytoin and three studies compared the former with either fosphenytoin or sodium valproate.
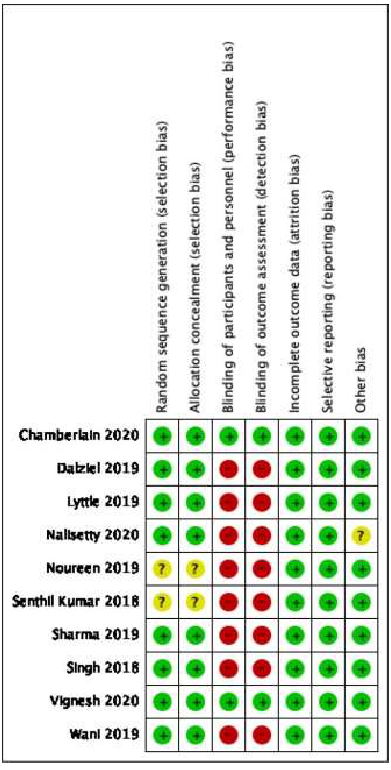
The risk of bias assessment was conducted for all the 10 studies under the following domains (Figure 1):
- Random sequence generation; 8 studies were deemed low risk of bias and 2 unclear
- Allocation concealment; 8 low risk, 2 unclear
- Blinding of participants and personnel; 8 high risk and 2 low risk
- Blinding of outcome assessment; 8 high risk and 2 low risk
- Incomplete outcome data; 10 low risk
- Selective reporting of data; 10 low risk
- Other bias; 9 low risk and 1 unclear.
Figure 1. Risk of Bias Assessment [5].
There was no significant difference in the clinical cessation of CSE in the 7 studies than compared levetiracetam to phenytoin (n=1640), there was moderate heterogeneity among these studies (RR=1.03, 95% CI 0.98 to 1.09) (I2=71%) [6-12].
Sensitivity analysis using a fixed random effects model yielded statistical significance in favor of levetiracetam (R=1.05, 95% CI 1.01 to 1.10). When the study that had an unknown risk of bias for random sequence generation and allocation concealment was excluded from the analysis then there was no difference in the results and the selected trials had low heterogeneity (RR=1.01, 95% CI 0.98 to 1.04) (I2=11%) (Figure 2).
No significant differences were found in the three studies that compared levetiracetam to fosphenytoin (n=267; RR=1.16, 95% CI 1.00 to 1.35; I2=7%) [13-15]. Similarly, no significant differences were found when levetiracetam was compared to sodium valproate (n=221; RR=0.41, 95% CI 0.15 to 1.13 I2=0%) [13,16].
Figure 2. Levetiracetam Vs Phenytoin: Seizure cessation[ 5].
Secondary outcomes
There was no significant difference in relation to the timing of the cessation of seizures between levetiracetam and phenytoin in the five studies where data was available (n=754, MD=-0.45, 95% CI -1.83 to 0.93; I2=87%) [7,9-12].
Similarly, no difference was found between the timing of cessation when levetiracetam was compared with fosphenytoin (n=111; MD=-0.70, 95% CI -4.26 to 2.86; I2=79%) [14,15].
Three studies that compared levetiracetam to phenytoin reported the need for rapid sequence intubation (RSI) and there was no difference between the two groups (n=586, RR=0.94, 95% CI 0.55 to 1.60; I2=50%) [6-7,11]. Similar results of no differences were found when levetiracetam was compared to fosphenytoin (n=267; RR=0.40, 95% CI 1.13 to 1.27; I2=29%) [13-15], and sodium valproate (n=221; RR=0.41, 95% CI 0.15 to 1.13) [15,16].
In terms of all-cause mortality, there was no difference when levetiracetam was compared to phenytoin in three of studies (n=585) (RR=0.57, 95% CI 0.07 to 4.62; I2=0%) [6-7,15] or when it was compared to sodium valproate in two of the trials (n=221; RR=0.57, 95% CI 0.07 to 4.59; I2=0%) [11,13].
Three studies compared levetiracetam to phenytoin with respect to recurrence of seizures within 24 hours and found no significant differences between the two groups (n=585) (RR=1.12, 95% CI 0.0.47 to 2.67; I2=77%) [9-10,12]. There was also no significant difference when levetiracetam was compared with fosphenytoin for seizure recurrence (n=111; RR=1.67, 95% CI 0.52 to 5.40; I2=0%) [14,15].
Subgroup analysis to determine the optimal dose of levetiracetam, four trials used doses of ≥ 40mg/kg/dose of levetiracetam and when compared with phenytoin found no significant difference between them (n=1223 RR=1.03, 95% CI 0.94 to 1.13; I2=79%) or with the two studies that reported on fosphenytoin (n=217; RR=1.21, 95% CI 0.92 to 1.59; I2=45%).
The quality of the evidence of the secondary outcomes was graded moderate because of the small size of some of the primary studies, inconsistency and heterogeneity of the studies.
Discussion
The authors recently published systematic review is the largest of its kind in the pediatric population with over 1900 children [5]. Levetiracetam compared well with phenytoin, fosphenytoin and sodium valproate with respect to termination of pediatric CSE and all the aforementioned secondary outcomes. Phenytoin was associated with a lower diastolic blood pressure relative to the levetiracetam group. The main side effect related to levetiracetam use was agitation [6].
Rapid sequence intubation (RSI) was more frequently encountered with fosphenytoin use compared with either levetiracetam or sodium valprate [13]. RSI can be associated with increased morbidity and increased health care costs which should not be dismissed at a time when there is a considerable pressure on health care resources.
A meta-analysis and systematic review by DeMott et al. [17] that focused on comparing phenytoin and levetiracetam showed no significant difference in efficacy of levetiracetam compared phenytoin. This was the largest review that had compared the two drugs at the time it was published and their review included both adults and children. The adverse in effects in both groups were reported to be low. There was a higher incidence of cardiac instability primarily hypotension in the phenytoin group (Levitracetam: 0.5% vs. Phenytoin: 1.9%).
A subgroup analysis of the pediatric age group within the same review also found no significant differences in the efficacy of levetiracetam when compared with phenytoin (5 RCTS’s included in the meta-analysis) [17].
In this review levetiracetam has been shown to be just effective as phenytoin, fosphenytoin and sodium valproate. The availability of three equally effective anticonvulsants enables clinicians the opportunity to tailor the choice of anticonvulsant to the specific needs of the patient especially if there are other comorbidities [18]. The quality of the evidence is graded as only moderate because of the degree of heterogeneity between the RCTSs included in the systematic review.
Levetiracetam offers several advantages such as ease and rapidity of administration where termination of the seizure at the earliest opportunity is the greatest priority for both health care professions and family members. In answer to our question…. Levetiracetam does add another arrow to the second line anticonvulsant quiver.
References
2. Trinka E, Höfler J, Leitinger M, Brigo F. Pharmacotherapy for status epilepticus. Drugs. 2015 Sep;75(13):1499-521.
3. Silbergleit R, Durkalski V, Lowenstein D, Conwit R, Pancioli A, Palesch Y, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. New England Journal of Medicine. 2012 Feb 16;366(7):591-600.
4. Zaccara G, Giorgi FS, Amantini A, Giannasi G, Campostrini R, Giovannelli F, et al., Tuscany study group on seizures in the emergency department and status epilepticus in adults. Why we prefer levetiracetam over phenytoin for treatment of status epilepticus. Acta Neurologica Scandinavica. 2018 Jun;137(6):618-22.
5. Abdelgadir I, Hamud A, Kadri A, Akram S, Pullattayil A, Akobeng AK, et al. Levetiracetam for convulsive status epilepticus in childhood: systematic review and meta-analysis. Archives of Disease in Childhood. 2021 May 1;106(5):470-6.
6. Lyttle MD, Rainford NE, Gamble C, Messahel S, Humphreys A, Hickey H, et al. Levetiracetam versus phenytoin for second-line treatment of paediatric convulsive status epilepticus (EcLiPSE): a multicentre, open-label, randomised trial. The Lancet. 2019 May 25;393(10186):2125-34.
7. Dalziel SR, Borland ML, Furyk J, Bonisch M, Neutze J, Donath S, et al. Levetiracetam versus phenytoin for second-line treatment of convulsive status epilepticus in children (ConSEPT): an open-label, multicentre, randomised controlled trial. The Lancet. 2019 May 25;393(10186):2135-45.
8. Noureen N, Khan S, Khursheed A, Iqbal I, Maryam M, Sharib SM, et al. Clinical efficacy and safety of injectable levetiracetam versus phenytoin as second-line therapy in the management of generalized convulsive status epilepticus in children: an open-label randomized controlled trial. Journal of Clinical Neurology. 2019 Oct 1;15(4):468-72.
9. Sharma P, Mandot S, Bamnawat S. Levetiracetam versus phenytoin for treatment of convulsive status epilepticus in pediatric population: a randomized controlled trial. Int J Contemp Pediatrics 2019;6:741–5.
10. Singh K, Aggarwal A, Faridi MM, Sharma S. IV levetiracetam versus IV phenytoin in childhood seizures: a randomized controlled trial. Journal of Pediatric Neurosciences. 2018 Apr;13(2):158.
11. Vignesh V, Rameshkumar R, Mahadevan S. Comparison of phenytoin, valproate and levetiracetam in pediatric convulsive status epilepticus: a randomized double-blind controlled clinical trial. Indian Pediatr. 2020 Mar 1;57.
12. Wani G, Imran A, Dhawan N, Gupta A, Giri JI. Levetiracetam versus phenytoin in children with status epilepticus. Journal of Family Medicine and Primary Care. 2019 Oct;8(10):3367.
13. Chamberlain JM, Kapur J, Shinnar S, Elm J, Holsti M, Babcock L, et al. Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial. The Lancet. 2020 Apr 11;395(10231):1217-24.
14. Nalisetty S, Kandasamy S, Sridharan B, Vijayakumar V, Sangaralingam T, Krishnamoorthi N. Clinical effectiveness of levetiracetam compared to fosphenytoin in the treatment of benzodiazepine refractory convulsive status epilepticus. The Indian Journal of Pediatrics. 2020 Feb 22:1-8.
15. Senthilkumar CS, Selvakumar P, Kowsik M. Randomized controlled trial of levetiracetam versus fosphenytoin for convulsive status epilepticus in children. Int J Pediatr Res. 2018;5(4):237-42.
16. Vignesh V, Rameshkumar R, Mahadevan S. Comparison of phenytoin, valproate and levetiracetam in pediatric convulsive status epilepticus: a randomized double-blind controlled clinical trial. Indian Pediatr. 2020 Mar 1;57.
17. DeMott JM, Slocum GW, Gottlieb M, Peksa GD. Levetiracetam vs. phenytoin as 2nd-line treatment for status epilepticus: a systematic review and meta-analysis. Epilepsy & Behavior. 2020 Oct 1;111:107286.
18. Smith PE. Management of established status epilepticus. New England Journal of Medicine. 2019 Nov 28;381(22):2171-2.