Abstract
Introduction: The primary aim of this study was to compare the accuracy of a point-of-care instrument for analysis of NT-proBNP (NT-proBNPLumiraDx) versus conventional plasma NT-proBNP results (NT-proBNPRoche). Secondary aims were: (i) to extract all plasma NT-proBNP results from this emergency department (ED) for the year 2022 and to characterize this patient cohort; (ii) to distinguish the coefficient of variation (CV) for NT-proBNP when analyzed with NT-proBNPLumiraDx.
Methods: In 142 patients NT-proBNPLumiraDx determinations were compared to NT-proBNPRoche. In 2022, a total of 8,333 NT-proBNP results were reported to the ED. These samples were routine requests for NT-proBNP from the ED at Uppsala University Hospital, Uppsala, Sweden. NT-proBNP was analyzed with the LumiraDx instrument in samples from 133 healthy blood donors, to calculate reference intervals.
Results: There was a significant Spearman rank correlation between age and NT-proBNPRoche analytes (P<0.0001). The median age of the patients was 76 years [interquartile range: (IQR) 65-84 years] and median NT-proBNP value was 576 ng/L (IQR: 138-2,390). In 3,612 out of 8,333 NT-proBNP test results were 400 ng/L or lower. The median CV for the NT-proBNPLumiraDx was 2.2% (range 0.1-5.2%).
Conclusion: There was a strong Deming correlation between the two NT-proBNP methods: NT-proBNPLumiraDx = 1.025x NT-proBNPRoche + 9.51; r = 0.982). NT-proBNP is an important analyte for the emergency department. The strong correlation between the two NT-proBNP assays indicates that the NT-proBNPLumiraDx could be used in the emergency department for point-of-care testing. This finding strongly supports the clinical importance of our findings.
Keywords
Emergency department, NT-proBNP, Method evaluation, Point-of-Care test, Heart failure
Abbreviations
NT-proBNP: N-Terminal pro B-type Natriuretic Peptide; HF: Heart failure; CV: Coefficient of variation; ED: Emergency Department; POC: Point of Care; NP: Natriuretic Peptides; IQR: Interquartile Range; CI: Confidence Interval; TAT: Test-turnaround Time
Introduction
Presently, more than 10% of patients >70 years of age suffer from heart failure (HF). As the older population increases in numbers and also as a share of the total population, the prevalence of HF will increase [1,2]. Untreated, approximately 60-70% of these patients will die within 5 years [3,4]. It is thus important to diagnose and treat HF patients early. Furthermore, NT-proBNP qualifies as a reliable surrogate marker of HF [5]. Although natriuretic peptides (NP) cannot be used to identify the origin of shock, they may have a prognostic value [6]. Point-of-care testing (POC) refers to any diagnostic test administered outside the central laboratory at or near the location of the patient, which indicates its clinical value as a rapid diagnostic tool.
Acute heart failure is the most frequent cause of acute hospital admissions in patients older than 65 years. Acute heart failure may be the first manifestation of heart failure (HF), or more often as an acute decompensation of chronic HF [7].
The most common symptoms of acute heart failure are dyspnea, orthopnea, and fatigue. However, these HF symptoms could also be due to a number of other conditions [8].
Cardiovascular biomarkers therefore play a crucial role in the diagnostic workup of acute heart failure patients. In dyspnoic patients arriving to the emergency department, NT-proBNP (or BNP) is frequently measured in order to differentiate between cardiac and non-cardiac causes of dyspnea [9].
Within minutes of synthesis, NT-proBNP is released into the circulation providing information on the overall cardiac load. A negative NT-proBNP can be used to rule out HF while an elevated NT-proBNP value is a strong indicator of heart failure [10,11].
The GUIDE-IT study in high risk patients with HF and reduced ejection fraction did not support a strategy of NT-proBNP guided therapy [12]. Still, a biomarker guided strategy aimed at decreasing NP levels, may improve outcomes in patients with chronic systolic HF, especially if there is an up-titration of diuretic therapy when NT-proBNP >5000 ng/L [13].
The primary aim of this study was to evaluate the LumiraDx POC NT-proBNP method by comparing the results with those obtained from Cobas Pro (Roche Diagnostics, Mannheim, Germany). Secondary aims were to determine NT-proBNP routine results during 2022 and to characterize this patient cohort. Also, we wanted to define the coefficient of variation (CV) for NT-proBNP when analyzed with the LumiraDx point of care instrument.
Methods
Study population
The samples used were routine requests for NT-proBNP from the emergency department at Uppsala University Hospital, Uppsala, which serves the county of Uppsala. The laboratory information system was used to extract patient results from January 1st, 2022 to December 31st, 2022. The county of Uppsala had 242,140 inhabitants in 2022. In total 8,333 NT-proBNP results were reported to the emergency department during 2022.
Furthermore, 142 samples were collected during February 2023 for method comparison between Cobas Pro (Roche Diagnostics, Mannheim, Germany) and LumiraDx (Solna, Sweden). The samples were collected in Lithium-heparin PST tubes (LH PST II tube 366567, Becton Dickinson, Franklin Lakes, NJ, USA) and initially analyzed on the Cobas Pro instrument at the Department of Clinical Chemistry and Pharmacology, Uppsala University Hospital, Sweden and then on the LumiraDx instrument.
We also analyzed NT-proBNP with the LumiraDx instrument in blood samples from 133 healthy blood donors (19-72 years, 49% females) to calculate reference intervals.
The local ethical committee (01-367) approved the collection of samples. The ethical permit limited the patient information to age and gender. The work was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
NT-proBNP assay comparison
Plasma NT-proBNP was measured at the Department of Clinical Chemistry, Uppsala University Hospital on a Roche Cobas Pro (Roche Diagnostics, Mannheim, Germany) according to the specifications of the manufacturer. The instrument had a total coefficient of variation (CV) of 4% at 120 ng/L and 4% at 1900 ng/L measured over three months.
As a method comparison, NT-proBNP was measured on the same tubes with the LumiraDx analyzer (LumiraDx, Solna, Sweden) according to the recommendations of the manufacturer. The total CV was 2.3% at 115 ng/L and 2.6% at 400 ng/L. The time interval between the measurements on the Cobas Pro and LumiraDx instruments was less than 6 hours. The assay volume on the LumiraDx instrument was 20 uL and the total assay time was 12 min.
Statistical analysis
The coefficient of variations and figures were prepared using Excel (Microsoft, Seattle, WA, USA). Deming correlations were calculated using Method Validator version 1.1 (Metz, France). Values below 50 ng/L (the lower detection limit for the LumiraDx analyser; n=3) were set to 50 ng/L in the analysis and values >9,000 were set to 9,000 (n=2) in the statistical analysis. A Bland-Altman plot was used to evaluate the agreement among the two NT-proBNP methods [14,15].
Results
Correlation between the two NT-proBNP methods
There was a strong Deming correlation between the two methods. NT-proBNPLumiraDx = 1.025 [95% confidence interval 0.962-1.088] x NT-proBNPRoche + 9.51 [-26.69-45.71]; r = 0.982. A slope value of 1.0 and an intercept of 0 were both within the 95% confidence intervals. A Bland-Altman plot was used to evaluate the correlation among the two NT-proBNP methods, where differences are shown as the differences between the two methods and their mean value (Figure1). In two patients LumiraDx showed maximal values (i.e. > 9.000 ng/L), whereas the Cobas Pro instrument exhibited values at 18,500 and 12,900 ng/L, respectively.
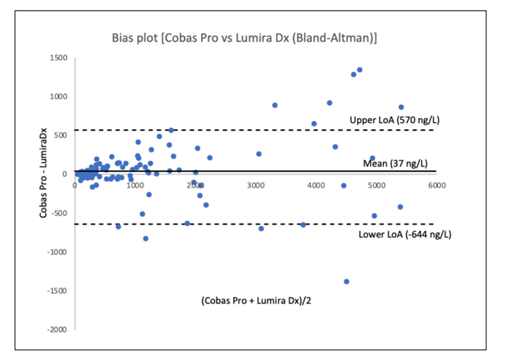
Figure 1: Bland-Altman plot showing validation between the Cobas Pro instrument and LumiraDx for determination of NT-proBNP (ng/L) in patients admitted to the emergency department at Uppsala University Hospital, Uppsala, Sweden. Limits of agreement (LoA) were calculated as mean difference ± 1.96 Χ standard deviation. In two patients the level of analysis for LumiraDx was exceeded, whereas considerably higher values were noted, when measured by the Cobas Pro instrument. These two values were not included in the plot.
NT-proBNP routine results reported to the emergency department during 2022
The number of NT-proBNP results reported to the emergency department during 2022 amounted to 8,333. Of these, 4,049 results were for females and 4,284 were for males. The median age of the patients was 76 years (IQR 65-84 years). Median NT-proBNP value for all emergency department samples was 576 ng/L (interquartile range (IQR) 138-2,390). There was a significant Spearman rank correlation between age and NT-proBNP value (rho= 0.548, 95% confidence interval (CI) 0.533-0.563, P<0.0001). Three thousand and twelve out of 8,333 NT-proBNP test results were 400 ng/L or lower.
Coefficient of variation for NT-proBNP with the LumiraDx analyzer
Three controls were analyzed daily (n=7). The total coefficient of variation (CV) was calculated for each of the three levels. The total CV was 2.3% at 114 ng/L, 2.6% at 402 ng/L and 4.4% at 1,080 ng/L.
Duplicate testing was performed for ten samples. The median CV for the ten samples was 2.2% (range 0.1-5.2%). There was a weak association between the NT-proBNP value and the CV (y = -1.03ln(x) + 7.88; R² = 0.224) (Table 1).
|
Mean NT-proBNP (ng/L) |
CV (%) |
|
64.95 |
3.93 |
|
103.4 |
3.09 |
|
113.4 |
0.13 |
|
123.6 |
5.18 |
|
189.7 |
3.56 |
|
262.1 |
1.49 |
|
324.2 |
2.56 |
|
349.3 |
1.93 |
|
393.1 |
0.31 |
|
686.8 |
1.70 |
Reference interval for NT-proBNP using the LumiraDx instrument
Lithium heparin blood samples from 133 healthy blood donors were analyzed. The calculated reference intervals were 50-165 ng/L for females, 50-103 ng/L for males, and 50-145 ng/L for both genders. 50 ng/L was the limit of detection for the LumiraDx instrument.
Discussion
NT-proBNP is an important clinical marker for HF. A strength with the NT-proBNP methods is that they are widely available, and several studies have shown that NT-proBNP is useful for triage, diagnosis, and prognostication of HF. A value of >300 ng/L is often used for the diagnosis of acute HF and a decrease of the value by >30% indicates response to decongestion therapy [10]. As the HF patients in the emergency department are relatively old and the NT-proBNP values increases with age, we calculated the proportion of patients with a NT-proBNP cut-off value of <400 ng/L [9,16].
POC shortens turn-around time (TAT), hereby giving the treating physician the opportunity to evaluate test results at the time of the visit as transport and laboratory processing times are abolished [17,18]. POC is also associated with improved patient satisfaction and greater levels of trust in their medical care as well as improved motivation to manage their own condition. Similar results were obtained in a randomized controlled clinical trial, where POC was evaluated [19,20].
NT-proBNP is a relatively new clinical biomarker, with a history of less than 20 years at our hospital. Despite this, the emergency department ordered >8,000 NT-proBNP results during 2022 making it one of the most expensive laboratory tests in the emergency department. The requests were mainly for elderly patients; this could be expected as the prevalence of HF increases with age [1]. The median value for the cohort was 576 ng/L. Although acute pulmonary embolism as well as both chronic thromboembolism pulmonary hypertension and chronic thromboembolic pulmonary disease may cause elevations in NT-proBNP [21,22], it seems reasonable to assume that the majority of these patients had suffered from HF.
The large number of requests verify that NT-proBNP is an important assay in the emergency department for the workup of patients. It has previously been shown that NT-proBNP testing is cost-effective in the emergency department [23,24]. To be used effectively, TAT should be short and the test should be available around the clock. A POC assay would shorten TAT at most emergency departments. We thus decided to evaluate the NT-proBNP assay from LumiraDx with emergency department samples. The comparison focused on samples in the 100-1000 ng/L range as this is frequently considered the most relevant one for treatment decisions [25-28]. The vast majority of our samples showed limited dispersions within this interval. The clinical relevance of the discrepancy between the estimations of NT-proBNP by the LumiraDx and the Cobas Pro instruments in the two patients with the highest values seems limited.
The LumiraDx instrument had a total CV of 2-5% at the three levels tested. It had a low variation for duplicate runs and showed a good agreement with the Cobas Pro. Further, the analyzer has advantages in being relatively small and easy to handle for the operator.
Circulating NT-proBNP and proBNP are O-glycosylated and may be present in different peptide fragments [29]. The glycosylation has been reported to have a negative effect on NT-proBNP recognition by the antibodies in the Roche assay [25,30]. This could potentially contribute to variations between the two studied methods. A strength of this study is the extensive number of individuals used for evaluation of the relation between NT-proBNPRoche and age in this cohort. Also, there was an expressed consistency between NT-proBNPLumiraDx and NT-proBNPRoche.
Our results may be in agreement with a previous study [26] on intra- and intersubject variability of POC NT-proBNP, suggesting that repeated determinations of this peptide may be of value in HF management.
A potential limitation of this study may be that body mass index is not taken into account, since obesity may have an impact on the interpretation of the clinical significance of determination of this peptide [31]. Another possible drawback is alterations in therapeutic interventions [32]. However, there is no reason to assume that various analytical methods should be an issue in either aspect.
Conclusions
NT-proBNP is an important analyte for the emergency department. The strong agreement between the two NT-proBNP assays, within a clinically relevant range, indicates that the LumiraDx NT-proBNP assay could be used in the emergency department for POC testing for rapid NT-proBNP results, facilitating adequate patient management at an earlier time point than otherwise would have been possible.
Acknowledgements
We are grateful to the scientific staff at Uppsala Unversity Hospital for assistance in running these assays. Mrs Margareta Hoerling is acknowledged for linguistic revision of the manuscript.
Funding
The project was supported by funding from the Uppsala University Research Fund. No other financial sources have contributed to this study.
Statement of competing interest
None of the authors declare any competing interests.
References
2. Wang H, Chai K, Du M, Wang S, Cai JP, Li Y, et al. Prevalence and Incidence of Heart Failure Among Urban Patients in China: A National Population-Based Analysis. Circ Heart Fail. 2021 Oct;14(10):e008406.
3. Stewart S, Ekman I, Ekman T, Odén A, Rosengren A. Population impact of heart failure and the most common forms of cancer: a study of 1 162 309 hospital cases in Sweden (1988 to 2004). Circ Cardiovasc Qual Outcomes. 2010 Nov;3(6):573-80.
4. Jhund PS, Macintyre K, Simpson CR, Lewsey JD, Stewart S, Redpath A, et al. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5.1 million people. Circulation. 2009 Feb 3;119(4):515-23.
5. Schmitt W, Rühs H, Burghaus R, Diedrich C, Duwal S, Eissing T, et al. NT-proBNP Qualifies as a Surrogate for Clinical End Points in Heart Failure. Clin Pharmacol Ther. 2021 Aug;110(2):498-507.
6. Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, et al. Heart Failure Association of the European Society of Cardiology. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019 Jun;21(6):715-31.
7. Metra M, Tomasoni D, Adamo M, Bayes-Genis A, Filippatos G, Abdelhamid M, et al. Worsening of chronic heart failure: definition, epidemiology, management and prevention. A clinical consensus statement by the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2023 Jun;25(6):776-91.
8. Masip J, Frank Peacok W, Arrigo M, Rossello X, Platz E, Cullen L, et al. Acute Heart Failure Study Group of the Association for Acute Cardiovascular Care (ACVC) of the European Society of Cardiology. Acute Heart Failure in the 2021 ESC Heart Failure Guidelines: a scientific statement from the Association for Acute CardioVascular Care (ACVC) of the European Society of Cardiology. Eur Heart J Acute Cardiovasc Care. 2022 Feb 8;11(2):173-85.
9. Maisel A, Mueller C, Adams K Jr, Anker SD, Aspromonte N, Cleland JG, et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008 Sep;10(9):824-39.
10. Núñez J, de la Espriella R, Rossignol P, Voors AA, Mullens W, Metra M, et al. Congestion in heart failure: a circulating biomarker-based perspective. A review from the Biomarkers Working Group of the Heart Failure Association, European Society of Cardiology. Eur J Heart Fail. 2022 Oct;24(10):1751-66.
11. Salzano A, D'Assante R, Israr MZ, Eltayeb M, D'Agostino A, Bernieh D, et al. Biomarkers in Heart Failure: Clinical Insights. Heart Fail Clin. 2021 Apr;17(2):223-43.
12. Felker GM, Ahmad T, Anstrom KJ, Adams KF, Cooper LS, Ezekowitz JA, et al. Rationale and design of the GUIDE-IT study: Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure. JACC Heart Fail. 2014 Oct;2(5):457-65.
13. Felker GM, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston-Miller N, et al. Effect of Natriuretic Peptide-Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA. 2017 Aug 22;318(8):713-20.
14. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986 Feb 8;1(8476):307-10.
15. Olofsen E, Dahan A, Borsboom G, Drummond G. Improvements in the application and reporting of advanced Bland-Altman methods of comparison. J Clin Monit Comput. 2015 Feb;29(1):127-39.
16. Webb J, Draper J, Rua T, Yiu Y, Piper S, Teall T, et al. A cost effectiveness study establishing the impact and accuracy of implementing the NICE guidelines lowering plasma NTproBNP threshold in patients with clinically suspected heart failure at our institution. Int J Cardiol. 2018 Apr 15;257:131-6.
17. Larsson A, Greig-Pylypczuk R, Huisman A. The state of point-of-care testing: a European perspective. Ups J Med Sci. 2015 Mar;120(1):1-10.
18. Kendall J, Reeves B, Clancy M. Point of care testing: randomised controlled trial of clinical outcome. BMJ. 1998 Apr 4;316(7137):1052-7.
19. Laurence CO, Gialamas A, Bubner T, Yelland L, Willson K, Ryan P, et al. Point of Care Testing in General Practice Trial Management Group. Patient satisfaction with point-of-care testing in general practice. Br J Gen Pract. 2010 Mar;60(572):e98-104.
20. Gialamas A, Yelland LN, Ryan P, Willson K, Laurence CO, Bubner TK, et al. Does point-of-care testing lead to the same or better adherence to medication? A randomised controlled trial: the PoCT in General Practice Trial. Med J Aust. 2009 Nov 2;191(9):487-91.
21. Dzikowska-Diduch O, Kurnicka K, Lichodziejewska B, Dudzik-Niewiadomska I, Machowski M, Roik M, et al. Electrocardiogram, Echocardiogram and NT-proBNP in Screening for Thromboembolism Pulmonary Hypertension in Patients after Pulmonary Embolism. J Clin Med. 2022 Dec 12;11(24):7369.
22. Cotugno M, Orgaz-Molina J, Rosa-Salazar V, Guirado-Torrecillas L, García-Pérez B. Right ventricular dysfunction in acute pulmonary embolism: NT-proBNP vs. troponin T. Med Clin (Barc). 2017 Apr 21;148(8):339-44. English, Spanish.
23. Siebert U, Milev S, Zou D, Litkiewicz M, Gaggin HK, Tirapelle L, et al. Economic Evaluation of an N-terminal Pro B-type Natriuretic Peptide-Supported Diagnostic Strategy Among Dyspneic Patients Suspected of Acute Heart Failure in the Emergency Department. Am J Cardiol. 2021 May 15;147:61-9.
24. Ferrandis MJ, Ryden I, Lindahl TL, Larsson A. Ruling out cardiac failure: cost-benefit analysis of a sequential testing strategy with NT-proBNP before echocardiography. Ups J Med Sci. 2013 May;118(2):75-9.
25. Halfinger B, Hammerer-Lercher A, Amplatz B, Sarg B, Kremser L, Lindner HH. Unraveling the Molecular Complexity of O-Glycosylated Endogenous (N-Terminal) pro-B-Type Natriuretic Peptide Forms in Blood Plasma of Patients with Severe Heart Failure. Clin Chem. 2017 Jan;63(1):359-68.
26. Chami J, Fleming S, Taylor CJ, Bankhead CR, James T, Shine B, et al. Point-of-care NT-proBNP monitoring for heart failure: observational feasibility study in primary care. BJGP Open. 2022 Sep 28;6(3):BJGPO.2022.0005.
27. Spinar J, Spinarova L, Malek F, Ludka O, Krejci J, Ostadal P, et al. Prognostic value of NT-proBNP added to clinical parameters to predict two-year prognosis of chronic heart failure patients with mid-range and reduced ejection fraction - A report from FAR NHL prospective registry. PLoS One. 2019 Mar 26;14(3):e0214363.
28. Lundgren M, Ridefelt P, Karlsson M, Norling A, Larsson A. Interlaboratory variation for NT-proBNP among Swedish laboratories in an external quality program 2011-2021. Clin Chem Lab Med. 2023 Mar 30;61(9):1643-51.
29. Amplatz B, Sarg B, Faserl K, Hammerer-Lercher A, Mair J, Lindner HH. Exposing the High Heterogeneity of Circulating Pro B-Type Natriuretic Peptide Fragments in Healthy Individuals and Heart Failure Patients. Clin Chem. 2020 Sep 1;66(9):1200-9.
30. Li L, Semenov AG, Feygina EE, Yang C, Wang N, Chen C, et al. Diagnostic utility of total NT-proBNP testing by immunoassay based on antibodies targeting glycosylation-free regions of NT-proBNP. Clin Chem Lab Med. 2022 Dec 2;61(3):485-93.
31. Madamanchi C, Alhosaini H, Sumida A, Runge MS. Obesity and natriuretic peptides, BNP and NT-proBNP: mechanisms and diagnostic implications for heart failure. Int J Cardiol. 2014 Oct 20;176(3):611-7.
32. Pieske B, Wachter R, Shah SJ, Baldridge A, Szeczoedy P, Ibram G, et al. PARALLAX Investigators and Committee members. Effect of Sacubitril/Valsartan vs Standard Medical Therapies on Plasma NT-proBNP Concentration and Submaximal Exercise Capacity in Patients With Heart Failure and Preserved Ejection Fraction: The PARALLAX Randomized Clinical Trial. JAMA. 2021 Nov 16;326(19):1919-29.
