Abstract
Background: Pain is a very important factor in patient recovery and satisfaction. Following incision and drainage of perianal abscess, wound packing has been found to be very painful. The aim of this study is to compare packing the wound and not packing the wound after incision and drainage (I&D) of perianal abscess, taking into account recurrence rate, rate of occurrence of perianal fistula as well as post-operative pain.
Materials and Method: Standard medical electronic databases were searched with the help of a local librarian and relevant published randomised controlled trials (RCT) were shortlisted according to the inclusion criteria. The summated outcome of post-operative pain score, recurrence rate, and rate of occurrence of perianal fistula was evaluated using the principles of meta-analysis on RevMan 5 statistical software.
Result: Three RCTs on 490 patients undergoing I&D of perianal abscess were found suitable for this meta-analysis. In the random effects model analysis, the post operative pain score was statistically lower in NPG [risk ratio 0.66, 95%, CI (0.36, 0.97), Z=4.25, P=0.0001]. There was no heterogeneity [Tau2=0.15; chi2=9.32; df=6; I2=36 %; p=0.16] between the studies, however, statistically it was not significant. In addition, there was no statistical difference between the 2 groups in terms of recurrence of perianal abscess or the occurrence of perianal fistula [risk ratio 0.64, 95%, CI (0.31, 1.31), Z=1.23, P=0.22], [risk ratio 1.41, 95%, CI (0.89, 2.23), Z=1.47, P=0.14] respectively.
Conclusion: Not packing the wound post I&D of perianal abscess is associated with significantly reduced VAS pain scores, which may result in improved recovery and patient satisfaction and has no effect on recurrence rate or the occurrence of perianal fistula. However, more RCTs recruiting a greater number of patients are required to endorse these findings.
Keywords
Perianal abscess, Fistula in ano, Wound packing, Incision and drainage (I&D), VAS pain score
Abbreviations
RCT: Randomized Controlled Trial; NHS: National Health Service; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; VAS: Visual Analogue Score; CI: Confidence Interval; RR: Risk Ratio; PG: Packing Group; NPG: Non-Packing Group
Introduction
An abscess is a localized collection of pus within a cavity surrounded by inflammation and granulation, as a reaction to an infectious source [1]. A perianal abscess is an abscess where the collection is confined to the perianal region. Most perianal abscesses are due to infection of the glandular crypts of the rectum and anus caused by non-specific obstruction [2]. A small percentage of cases, around 10%, can be attributed to alternative causes such as Crohn's disease, trauma, human immunodeficiency virus, sexually transmitted diseases, radiation therapy, or foreign bodies [3]. Perianal abscesses are considered one of the most common colorectal pathologies with an estimated annual incidence between 14,000 and 20,000 people in the UK, resulting in about 12,500 operations in the NHS each year [4]. Anorectal abscesses can spread into the ischiorectal fossa and can lead to a horse?shoe shaped collection or track up towards and through the levator musculature [5,6], which can make the management more challenging. Perianal fistulae are a common complication of peri-anal abscesses with a mean incidence of 8.6 per 100,000 [7]. Fistulae can be classified according to their tract location in relation to the internal and external sphincters as transphincteric fistula, high intersphincteric fistula, suprasphincteric fistula or extrasphincteric fistula [8].
Acute perianal pain is the most common presentation of perianal abscesses, which may increase with defecation, movement, sitting or coughing. Supralevator abscesses may present with lower back pain or a dull ache in the pelvic region. Patients may also report fever, malaise, rectal drainage, erythema of surrounding skin and possibly urinary retention [9]. The severity of pain can sometimes prevent the clinician from performing a digital rectal examination or anoscopic examination, therefore if the diagnosis is in doubt, an examination under general anesthesia should be performed [3]. In the presence of a perianal fistula, imaging modalities such as CT scans are helpful in diagnosing intraabdominal pathology such as Crohn’s disease and detecting air within the fistulous tract and the abscess cavity. However, MRI is the investigation of choice in evaluating secondary extensions from the fistulous tract and differentiating it from nearby pelvic soft tissue structures [10].
The management of perianal abscesses is incision and drainage. Without adequately eliminating the source of infection, antibiotics will be ineffective [11]. Minimizing the patient’s pain, protecting anal sphincter function and reducing the recurrence of anal fistulae is as important as curing the abscess [3,12]. Packing at the time of abscess drainage, which requires multiple dressing changes per week for several weeks, can be helpful in providing hemostasis of the inflamed, hypervascular abscess cavity [6,13].
Pain is defined as unpleasant sensory and emotional experience associated with actual and potential damage or described in terms of such damage [14]. Inadequate pain-relief results in an increase in length of stay, time to discharge, readmission rates, and time before ambulation, all of which can increase the cost of care [15]. Therefore, pain is a very important factor when it comes to patient recovery and satisfaction and wound packing has been found to be very painful, with a twofold increase in pain measured by visual analogue score (VAS) during a dressing change [13]. The aim of this study is to compare packing the wound and not packing the wound after incision and drainage of perianal abscess, taking into account recurrence rate and rate of occurrence of perianal fistula, as well as post-operative pain.
Methods
Data sources and literature search technique
Standard electronic databases including MEDLINE, EMBASE, and Cochrane Library were used to conduct a literature search identifying suitable articles for this meta-analysis. MeSH search terms found in the Medline library related to the target objective were used to find the relevant randomized controlled and retrospective trials. Limits for language, gender, sample size, and place of study origin were not applied, and these characteristics were recorded. The search results were narrowed and widened with use of Boolean operators (AND, OR, NOT). The titles from the search results were carefully inspected and found to be appropriate for potential inclusion or exclusion. The references used in chosen articles were also scrutinized as a strategy to identify any further trial for potential inclusion.
The inclusion criteria
To be included in this meta-analysis, all studies were required to give comparison of post operative pain, abscess recurrence rate and, rate of perianal fistula formation between packing or not packing the wound following incision and drainage of perianal abscess.
Collection of the data
Two different reviewers used a predefined meta-analysis data extraction form to obtain all the reported data. This was matched and was found to be in reasonable inter-reviewer agreement. The extracted data consisted of a list of the authors, title of the published study, journal of publication, country and year of the publication, testing sample size (with sex differentiation if applicable), the number of patients in each group based on packing the wound or not, protocol for each intervention, postoperative pain score, recurrence rate, and the presence of perianal fistula. The independent reviewers thoroughly discussed the data following extraction and if any differences were found, a mutual agreement was reached.
Statistical synthesis of the collected data
The software package RevMan 5 (The Nordic Cochrane Centre, Copenhagen, Denmark) [16,17] provided by the Cochrane Collaboration was used for statistical analysis. The risk ratio (RR) with a 95 per cent confidence interval (CI) was used to present the summated outcome for binary data. The combined outcomes were calculated using the random-effects model [18,19]. The chi2 test was used to explore heterogeneity among the included studies, with significance set at p<0.05. The I2 test was used to quantify this, with a maximum value of 30 percent identifying low heterogeneity [20]. To calculate RR under the random effect model [21] analysis, the Mantel-Haenszel method was used.
When conducting sensitivity analysis for trials in which no event occurred in either the treatment or control group, 0.5 was added to each cell frequency as recommended by Deeks et al. [22]. When no standard deviation was available, it was calculated from the p-value according to guidelines provided by the Cochrane Collaboration [18]. In this process it was assumed that both groups had the same variance, which may not have been true, and variance was estimated from either the range or the p-value. The estimated difference between both techniques was pooled depending upon the effect weights in results, which was determined by each trial estimate variance. The results were displayed graphically using a forest plot. The accuracy of the estimation (sample size) was represented by the square around the estimate, and the 95% CI by the horizontal line. Guidelines published by Jaddad et al, Chalmers et al., and Rangel et al. [23-25] were used to assess the methodological quality of included trials. For the purpose of analysis, the VAS score used to assess pain was divided by 10.
Primary endpoint
Post-operative pain was examined as the primary endpoint in this meta-analysis comparing packing group [PG] versus non-packing group (NPG).
Secondary endpoints
Recurrence of perianal abscess and the presence of fistula-in-ano were analyzed as the secondary endpoints.
Results
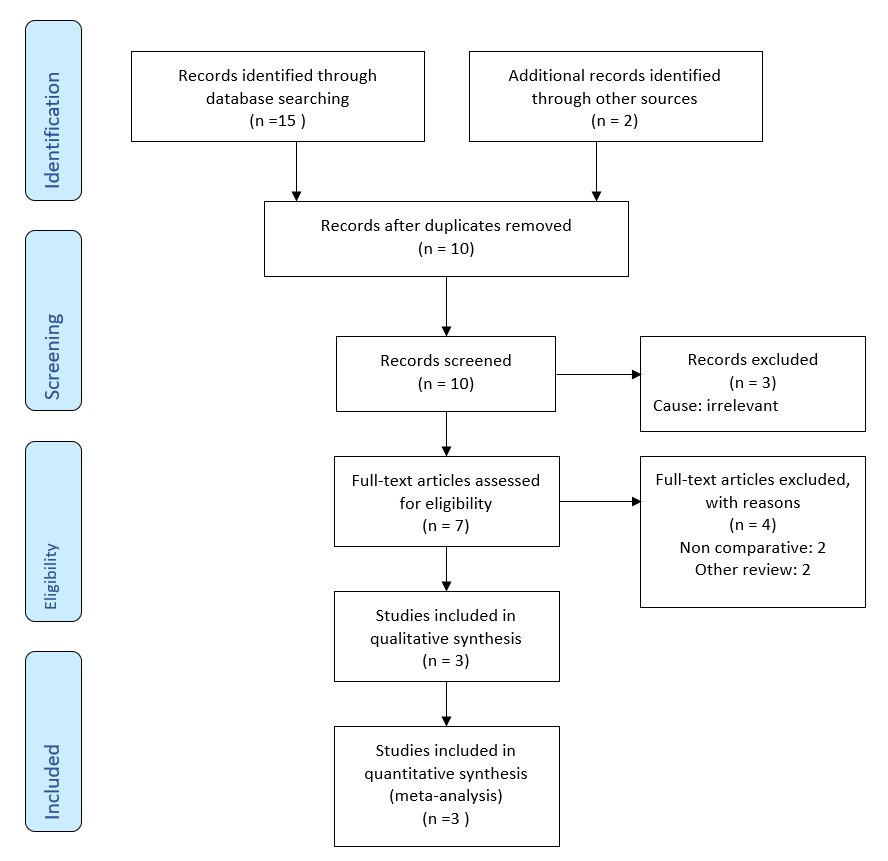
After removal of duplicated studies, the literature search generated 10 studies. 3 studies were considered irrelevant following the assessment of titles and abstracts of all these studies. After further examination of the remaining 7 studies, just 3 were found to be eligible for inclusion in the systematic review (Figure 1).
Figure 1. Prisma flow chart showing literature search outcomes.
Qualities of studies and patients
Based upon the principles provided by the Cochrane Collaboration, three RCTs [26-28] on 490 patients fulfilled the inclusion criteria to conduct this meta-analysis. The PRIMA flow chart in trial search, trial deletion, trial selection and inclusion are given in Figure 1. The included trials were conducted in the UK [26,27] and Australia [28]. The number of patients involved varied between the trials, ranging from 14 [27] to 433 [26]. All trials were conducted between 2004 [28] and 2022 [26]. In the non-packing group, external absorbent dressings or pads or superficial protective dressings were used, while the packing material was not mentioned in all three trials. There was no discrimination for study selection in terms of gender, age, number of recruited patients or language of the published study. The main characteristics of the included studies are given in Table 1 and the treatment protocol adopted in each of the studies is given in Table 2.
|
Study |
Year |
Country |
Number of Patients |
Male to Female Ratio |
Mean Age |
|
Newtown |
2022 |
UK |
433 |
M 289 : F 144 |
42 (31–52) |
|
Perera |
2014 |
UK |
14 |
M 7 : F 7 |
47.00 |
|
Tonkin |
2004 |
Australia |
43 |
M 35 : F 8 |
32.75 |
|
Study |
Type of Surgery |
Intervention (non-packing group) |
Control (packing group) |
|
Newtown |
Not mentioned |
external absorbent dressings or pads |
Packing material not mentioned |
|
Perera |
I&D of the abscess |
superficial protective dressing |
Packing material not mentioned |
|
Tonkin |
I&D of the abscess |
superficial protective dressing |
Packing material not mentioned |
Methodological evaluation of included studies
The methodological quality of included trials is summarized in Table 3. The Mantel-Haenszel random effects model was used to compute robustness and susceptibility to any outlier among these trials. Two of the randomized trials used a treatment allocation randomization system and computer-generated randomization sequence [26,27], and concealment was achieved using opaque envelops in two trials [27,28] while concealment was not mentioned in one study [26]. Blinding was not reported in just one of the randomized trials [28].
|
Study |
Randomization technique |
Blinding |
Concealment |
Intention to treat |
|
Newtown |
Treatment Allocation Randomization System (TARDIS) |
Assessor blind |
Not reported |
Reported |
|
Perera |
computer-generated randomization sequence |
Un-blinded |
Sealed Envelop |
Reported |
|
Tonkin |
Not reported |
Not reported |
Sealed Envelop |
Reported |
The outcome of the primary variable
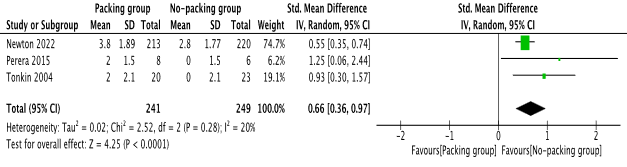
In the random effects model analysis, the post operative pain score was statistically lower in NPG [risk ratio 0.66, 95%, CI (0.36, 0.97), Z=4.25, P=0.0001]. There was no heterogeneity [Tau2=0.15; chi2=9.32; df=6; I2=36%; p=0.16] between the studies, however, statistically it was not significant. The individual risk ratio (RR) and summated RR with 95 per cent confidence intervals for the random effects model meta-analysis of included studies are presented in Figure 2.
Figure 2. Forest plot showing the post operative VAS pain score after incision and drainage of perianal abscess. The outcome is presented as risk ratio with 95% confidence interval.
The outcome of secondary endpoints
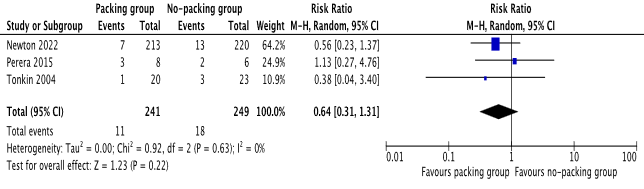
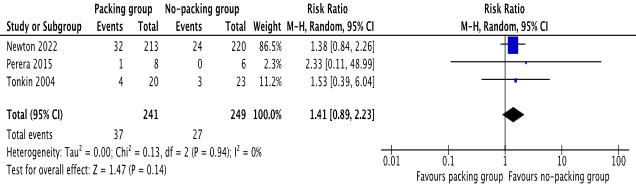
There was no statistical difference between the 2 groups in terms of recurrence of perianal abscess or the occurrence of perianal fistula [risk ratio 0.64, 95%, CI (0.31, 1.31), Z=1.23, P=0.22], [risk ratio 1.41, 95%, CI (0.89, 2.23), Z=1.47, P=0.14] respectively. Please see Figure 3 and Figure 4 for the individual risk ratio (RR) and summated RR with 95 per cent confidence intervals.
Figure 3. Forest plot showing recurrence of perianal abscess after incision and drainage. The outcome is presented as risk ratio with 95% confidence interval.
Figure 4. Forest plot showing occurrence of fistula in ano after incision and drainage. The outcome is presented as risk ratio with 95% confidence interval.
Discussion
This systematic review of three RCTs on 490 patients undergoing incision and drainage of perianal abscess indicated that not packing the wound post operatively is associated with significantly reduced VAS pain scores, which may result in improved recovery and patient satisfaction. In the random effects model analysis, the VAS pain score was found to be significantly lower in the non-packing group and there was no heterogeneity between the included studies.
In addition, there was no statistically significant difference between the groups in the recurrence of perianal abscess or the presence of perianal fistulae. This suggests that packing the wound post-operatively does not provide any benefit in terms of these unfavorable outcomes, despite causing significant pain to the patient.
The finding of a previously published review [29] is consistent with the finding of this current meta-analysis. Due to the lack of RCTs, the previous meta-analysis analyzed retrospective studies alongside RCTs which can generate bias. To the best of our knowledge, this is the only meta-analysis which consists of only RCTs reporting the effectiveness of not packing the wound post incision and drainage of perianal abscess to reduce post operative pain. It provides relatively strong evidence to consider not packing the wound in patient undergoing incision and drainage.
The main limitation of this study is the lack of RCTs and the use of only 490 patients. A multicenter larger RCT is required to solidify the results of this study before the standard recommendation of not packing of the wound following incision and drainage of perianal abscess. Moreover, future studies ought to compare the healing time in both groups in order to identify the optimal post operative management of perianal abscess.
Conclusion
VAS pain scores post I&D of perianal abscess was found significantly reduced when the wound was not packed. This can result in a better patient satisfaction, improved recovery and has no effect on recurrence of perianal fistula. However, more RCTs recruiting a greater number of patients are required to validate these finding.
Acknowledgments
None.
Conflict of Interest
None.
Funding
None.
References
2. Sigmon DF, Emmanuel B, Tuma F. Perianal Abscess. Treasure Island (FL): StatPearls Publishing; 2022 Jun 21.
3. Whiteford MH. Perianal abscess/fistula disease. Clin Colon Rectal Surg. 2007;20(2):102-109.
4. NHS Digital. Hospital Episode Statistics admitted patient care—England, 2014-2015. www.hscic.gov.uk/hes.
5. Read DR, Abcarian H. A prospective survey of 474 patients with anorectal abscess. Dis Colon Rectum. 1979;22:566‐568.
6. Whiteford MH, Kilkenny J, Hyman N, Buie WD, Cohen J, Orsay C, et al. Practice parameters for the treatment of perianal abscess and fistula‐in‐ano (revised). Dis Colon Rectum. 2005;48:1337‐1342.
7. Sainio P. Fistula-in-ano in a defined population. Incidence and epidemiological aspects. Ann Chir Gynaecol. 1984;73(4):219-224.
8. Jimenez M, Mandava N. Anorectal Fistula. Treasure Island (FL): StatPearls Publishing; 2022.
9. Turner SV, Singh J. Perirectal Abscess. Treasure Island (FL): StatPearls Publishing; 2022.
10. Balcı S, Onur MR, Karaosmanoğlu AD, Karçaaltıncaba M, Akata D , Konan A , et al. MRI evaluation of anal and perianal diseases. Diagn Interv Radiol. 2019;25(1):21-27.
11. Choi YS, Kim DS, Lee DH, Lee JB, Lee EJ, Lee SD, et al. Clinical Characteristics and Incidence of Perianal Diseases in Patients With Ulcerative Colitis. Ann Coloproctol. 2018 Jun;34(3):138-143.
12. Amato A, Bottini C, De Nardi P, Giamundo P, Lauretta A, Realis Luc A, et al. Evaluation and management of perianal abscess and anal fistula: SICCR position statement. Tech Coloproctol 2020;24:127-43.
13. Pearce L, Newton K, Smith SR, Barrow P, Smith J, Hancock L, et al. Multicentre observational study of outcomes after drainage of acute perianal abscess. Br J Surg. 2016;103:1063-1068.
14. Merskey H, Albe Fessard D, Bonica JJ, Carmon A, Dubner R, et al. Pain terms: a list with definitions and notes on usage. Recommended by the IASP subcommittee on taxonomy. PAIN. 1979;6:249-252.
15. Twersky R, Fishman D, Homel P. What happens after discharge? Return hospital visits after ambulatory surgery. Anesth Analg. 1997;84:319-324.
16. Harrower HW. Isolation of incisions into body cavities. Am J Surg. 1968;116 (6):824-826.
17. Cochrane Handbook for Systematic Reviews of Interventions | Cochrane Training [Internet]. [cited 2021 Oct 4]. Available from: https://training.cochrane.org/handbook
18. RevMan 5 download | Cochrane Training [Internet]. [cited 2021 Oct 4]. Available from: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download
19. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177-88.
20. DL D. Methods for combining randomized clinical trials: strengths and limitations. Statistics in medicine. 1987;6(3):341-8.
21. JP H, SG T. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002 Jun 15;21(11):1539-58.
22. Egger Matthias, Smith GDavey, Altman DG. Systematic reviews in health care: meta-analysis in context. 2001;487.
23. Deeks JJ, Altman DG, Bradburn MJ. Statistical Methods for Examining Heterogeneity and Combining Results from Several Studies in Meta-Analysis. Systematic Reviews in Health Care: Meta-Analysis in Context. Second Edition. 2008; pp. 285-312.
24. Jaddad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12.
25. Chalmers TC, Smith H Jr, Blackburn B, Silverman B, Schroeder B, Reitman D. A method for assessing the quality of a randomized control trial. Control Clin Trials. 1981;2:31-49.
26. Newton K, Dumville J, Briggs M, Law J, Martin J, Pearce L, et al. Postoperative Packing of Perianal Abscess Cavities (PPAC2): randomized clinical trial. Br J Surg. 2022 Sep 9;109(10):951-957.
27. Perera AP, Howell AM, Sodergren MH, Farne H, Darzi A, Purkayastha S, et al. A pilot randomised controlled trial evaluating postoperative packing of the perianal abscess. Langenbecks Arch Surg. 2015 Feb;400(2):267-271.
28. Tonkin DM, Murphy E, Brooke-Smith M, Hollington P, Rieger N, Hockley S, et al. Perianal abscess: a pilot study comparing packing with nonpacking of the abscess cavity. Dis Colon Rectum. 2004 Sep;47(9):1510-1514.
29. Smith SR, Newton K, Smith JA, Dumville JC, Iheozor-Ejiofor Z, Pearce LE, et al. Internal dressings for healing perianal abscess cavities. Cochrane Database Syst Rev. 2016 Aug 26;2016(8):CD011193.